ADA 2020 Highlights from Day 4 and Day 5
By Jimmy McDermottEliza Skoler
 By Jimmy McDermott, Eliza Skoler, Matthew Garza, Divya Gopisetty, Frida Velcani, Emily Fitts, and Karena Yan
By Jimmy McDermott, Eliza Skoler, Matthew Garza, Divya Gopisetty, Frida Velcani, Emily Fitts, and Karena Yan
ADA 2020 is officially in the books! Here are some more of our highlights from the last day and a half of ADA’s virtual 80th Scientific Sessions: Teplizumab, Control-IQ, SGLT-2s and GLP-1s, time in range, food as medicine, and more!
Our team wrapped up our coverage of ADA’s 2020 Scientific Sessions. Below you will find highlights from Day #4 and Day #5, including medicines that show promise for preventing diabetes-related health complications (and even diabetes onset!), real-world Control-IQ data, a functional food as medicine program, and the effects of health policy on diabetes care.
Click to jump to a section:
-
New Data Shows Teplizumab Delays Diagnosis of Type 1 Diabetes
-
Tandem’s Control-IQ Real-World Data: Time in Range Increases 2.4 Hours Per Day
-
A1C vs. Time in Range – Which Should be Used for Children with Diabetes?
-
SGLT-2 Inhibitors and GLP-1 Agonists to Prevent Heart Disease
A huge thanks to all the researchers, healthcare professionals, diabetes advocates, conference organizers, and attendees who helped create such intriguing virtual Scientific Sessions. You can read our coverage from day 1, day 2, and day 3, and stay tuned for our upcoming article with all of the highlights from this year’s conference!
New Data Shows Teplizumab Delays Diagnosis of Type 1 Diabetes
At last year’s ADA, we were very excited to report on trial results that showed teplizumab (pronounced Tep-pli-ZU-mab!) delayed type 1 diabetes diagnosis by two years, compared to placebo. The study enrolled 76 participants (55 children and 21 adults) who were the relatives of people with type 1 diabetes and did not have diabetes, and were at high risk for developing the condition (they had unstable blood glucose levels and at least two diabetes-related antibodies). On average, time to diagnosis of type 1 diabetes for the teplizumab group was four years, compared to two years with placebo. At the end of the trial, 53% of the teplizumab-treated group did not have type 1 diabetes, compared to 28% of the placebo group.
New follow up data, presented by Dr. Emily Sims (Indiana University), showed sustained reduction in the onset of type 1 diabetes. Previously, teplizumab had been proven to delay clinical onset by only two years in high-risk people; however, these new data support a delay of as much as three years, compared to placebo.
Furthermore, people who were treated with teplizumab showed a “striking reversal” in C-peptide decline (this is a common measure of type 1 diabetes) in the six months following treatment, after which C-peptide levels seemed to stabilize. These data suggest that the treatment helped stabilize beta cell function (the cells in the pancreas that make insulin) and that repeated teplizumab treatment at key time points may be able to further extend, delay, or even prevent diagnosis of type 1 diabetes. While not a cure, three years of living without daily diabetes management is certainly a meaningful outcome.
When will teplizumab become available? With an estimated six-month review time if Priority Review is granted, an FDA decision could be expected as soon as mid-2021.
Tandem’s Control-IQ Real-World Data: Time in Range Increases 2.4 Hours Per Day
Tandem presented two posters featuring very positive real-world data from early Control-IQ users. Control-IQ was cleared in December 2019 and officially launched in January 2020.
The first poster, Control-IQ Technology in the Real World: The First 30 days, included at least 30 days of pre- and post-Control-IQ data from 1,659 participants. During the first 30-days of Control-IQ use:
-
Time in range increased by 2.4 hours a day (compared to pre-Control-IQ data) to 78%
-
The time in range improvement was driven by a 9.5% decrease in time spent above 180 mg/dl (that’s 2.3 hours less per day in hyperglycemia – wow!).
-
Average glucose levels fell from 161 mg/dL to 148 mg/dL.
-
Glucose management indicator (or GMI, an estimate of A1C) fell from 7.2% to 6.9%.
-
Users spent 96% of time in closed loop!

The second poster, Glycemic Outcomes for People with Type 1 and Type 2 Diabetes Using Control-IQ Technology: Real-World Data from Early Adopters, looked at 2,896 participants with type 1 diabetes and 144 participants with type 2 diabetes, using at least 14 days of pre- and post-Control-IQ data.
-
Time in range was improved by 2.1 hours per day in the type 1 group to 77%
-
Time in range was improved by 1.4 hours per day in the type 2 group 79%
-
Both groups spent 96% of time in closed loop.
We learned so much at ADA about improving time in range, and we were moved by the power of automated insulin delivery in doing so, since it shows much greater time in range with what sounds like so less work for people and their healthcare teams.
To learn more about Control-IQ, check out the following articles:
A1C vs. Time in Range – Which Should be Used for Children with Diabetes?
A panel discussion of leading experts, moderated by JDRF CEO Dr. Aaron Kowalski, focused on the pros and cons of using A1C and time in range as primary metrics in diabetes care and management for children. As they debated the best marker of glucose management, they attempted to define the ultimate “goal” of diabetes care: is it preventing complications, spending less time in hyperglycemia and hypoglycemia, or improving mental and emotional wellbeing?
Dr. William Winter presented extensive evidence that A1C can predict a person’s risk of developing complications (kidney disease, heart disease, retinopathy, and neuropathy). While lower time in range has been associated with microvascular complications, experts agree that more studies are needed to determine its predictive accuracy for long-term outcomes. Dr. Thomas Danne presented results from the SWEET project that furthered the case for A1C as a measure of population outcomes: setting ambitious targets based on A1C could lead to significant improvements in outcomes for children with type 1 diabetes.

Experts discussed cases in which A1C can be misleading and time in range may emerge as a more reliable measure of glucose control. Dr. Winter explained that population A1cs differ among racial and ethnic groups, leading to misdiagnosis (for example, African Americans have a higher A1c on average compared to white people). Very importantly, as diaTribe has reported on for many years in Beyond A1C research, A1C also does not demonstrate hypoglycemia, hyperglycemia, or glucose variability. According to Dr. Danne, healthcare professionals find CGM reports more helpful in identifying daily highs and lows and in adjusting therapy. This technology allows them to better work alongside families to set individual and measurable goals based on time in range – it is terrific to hear about this continued teamwork.
.jpg)


Though Dr. Danne acknowledged the issue of access and affordability, he believes CGM use will continue to increase among children who are tech savvy. Dr. Daniel DeSalvo presented data from the SENCE and CITY to further support use of CGM among children with type 1 diabetes. Young children (two to seven years old) enrolled in the SENCE study saw their hypoglycemia (blood glucose under 70 mg/dL) and time spent over 300 mg/dL reduce by 40 minutes per day – that’s nearly five hours a week. Teens and young adults (ages 14 to 24) in the CITY study saw a 7% increase in time in range, which is almost two more hours per day spent in range – 100 minutes, to be exact!
Effects of Health Policy on Diabetes Care
Professor Rebecca Myerson (from the University of Wisconsin) shared key findings of a study on the impact of Medicaid expansion for people with diabetes:
-
Medicaid prescriptions for insulin increased by about 40%, even with rising insulin prices, meaning that more people with diabetes are receiving treatment.
-
Prescriptions for metformin also increased, suggesting that more people are getting treatment for early-stage diabetes.
-
About one-third of the other prescriptions are for newer medicines (such as SGLT-2 inhibitors and GLP-1 agonists) – promising trends for preventing diabetes complications and saving significant costs down the road.
Dr. Kasia Lipska from Yale School of Medicine discussed the importance of coverage for essential medicines and pre-existing conditions – two health policy issues that are front of mind for many Americans as the November election approaches. In addition to Medicaid expansion, the Affordable Care Act (ACA, or Obamacare) provided coverage for “Essential Health Benefits,” which includes prescription drugs, mental health services, emergency services and hospital care, preventive services and chronic disease management, and more. Dr. Lipska shared a study that found the ACA reduced the percent of income spent on family medical costs for people ages 18-64 with diabetes. This reduction was especially true for people whose family income was in the lowest bracket ($0-34,999 per year).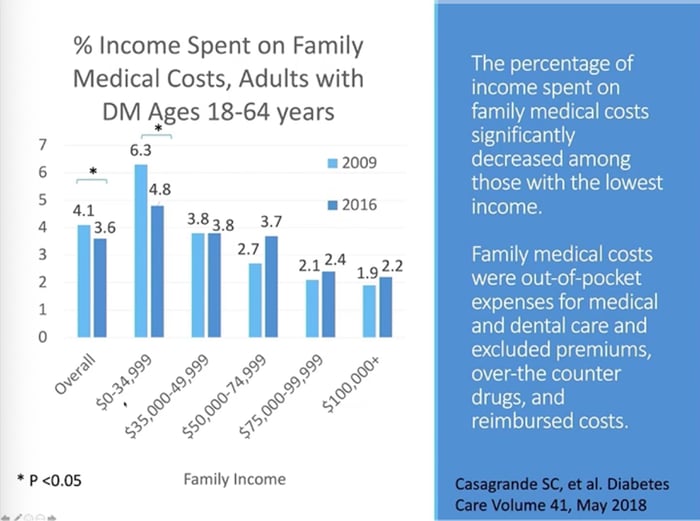
Importantly, ACA also prohibited health insurance companies from denying people coverage or charging higher costs to people who have “pre-existing conditions,” including diabetes. Given the significant improvements in coverage and care, Dr. Lipska emphasized that getting rid of the pre-existing conditions provisions would be “a disaster for people with diabetes” – presumably diaTribe readers in the US would agree! Over half of those surveyed were in favor of expanding Medicaid programs in their state – this doesn’t surprise us, since there are so many states that do not have favorable diabetes care programs (for example, see our article on CGM coverage for people on Medicaid; although this was not part of the ACA, many cite it as helping improve care quickly for those that are able to access the benefit). She shared results of a Kaiser Family Foundation survey that emphasized the need for ACA provisions:.png)
SGLT-2 Inhibitors and GLP-1 Agonists to Prevent Heart Disease
Dr. Mikhail Kosiborod (University of Missouri-Kansas City) and Dr. Darren McGuire (University of Texas Southwestern Medical Center) debated the use of SGLT-2 inhibitors and GLP-1 agonists in primary prevention of heart disease (called cardiovascular disease, or CVD).
As background, primary prevention is using medication in people who do not have CVD in order to prevent CVD. This is different from secondary prevention in which a person who is diagnosed with CVD uses a medication to prevent progression of the disease.
Dr. Kosiborod started the session with a strong “yes” – SGLT-2 inhibitors and GLP-1 agonists should be used for primary prevention. However, primary prevention is difficult to prove: larger and longer trials are needed. Dr. Kosiborod believes that we do have enough evidence.
-
A meta-analysis of SGLT-2 inhibitor trials suggests that:
-
SGLT-2 therapy works to prevent heart failure regardless of whether a person has established CVD (based on hospitalizations for heart failure).
-
SGLT-2 therapy protects kidney health regardless of whether a person has established CVD.
-
-
The FDA has approved SGLT-2 inhibitor Farxiga for people with type 2 diabetes and established CVD, and those with risk factors for CVD. That is primary prevention!
-
REWIND showed that GLP-1 agonist Trulicity prevents major adverse cardiovascular events (MACE, which includes stroke, heart attack, and cardiovascular death) in people with and without established CVD.
-
The FDA agrees again here – Trulicity is approved for people with type 2 diabetes with CVD and those with risk factors for CVD.
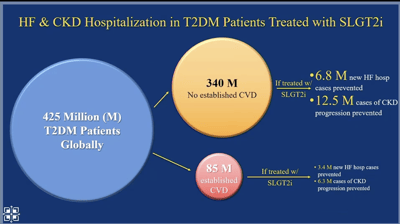
Next, Dr. Kosiborod looked at the population level. Worldwide, primary prevention with SGLT-2s and GLP-1s will significantly reduce cardiovascular events (compared to secondary prevention alone) because there are many people who are not diagnosed with CVD.
Dr. Kosiborod believes this primary prevention is cost-effective and essential, given the high risk to the population. And many SGLT-2s and GLP-1s will become generic in the future.
Dr. McGuire argued that we are not ready for SGLT-2s and GLP-1s to be used in primary prevention. He pointed to a meta-analysis that showed no benefit of SGLT-2 inhibitors and GLP-1 agonists in atherosclerotic cardiovascular disease (ASCVD) outcomes compared to placebo in people without established ASCVD. In his analysis of REWIND, Dr. McGuire pointed to an absolute risk difference of 0.3% in people without established CVD taking Trulicity versus placebo (1.7 events for every 100 patient years, vs. 2.0 events for every 100 patient years). This would mean that you would need to treat 333 people without CVD to prevent one MACE – which would be $3.4 million in drug costs.
Both speakers agreed that SGLT-2 inhibitors have shown strong effects in primary prevention for heart failure and kidney outcomes. There was no significant debate on this point, as the data speak for themselves regarding the profound effect of SGLT-2 treatment in reducing these outcomes.
Weekly Basal Insulin – The Wave of the Future?
New types of insulin – once-weekly basal insulin injections – are being tested in clinical trials and may bring major developments to how people take insulin. In this session, Professor Philip Home, Dr. J. Hans DeVries, and Dr. Stefano Del Prato discussed the pros and cons and recent results from clinical trials of weekly basal insulin.
Prof. Home explained that weekly insulin could reduce hurdles in starting or maintaining insulin therapy for people with diabetes, especially those who are:
-
Afraid of injections
-
Hesitant to start insulin due to the change in lifestyle or impact on quality of life
-
Wary about handling devices
-
Already on a weekly injectable GLP-1 agonist
Weekly insulin could help people adhere to their prescribed therapy – but it will likely make dose titration and adjustments more challenging. One of the major challenges of weekly insulin is that people can’t modify insulin doses according to life disruptions (for example, sick days or increased physical activity).
Dr. DeVries and Dr. Del Prato reviewed the various weekly insulins that companies are studying to evaluate their safety and how they affect diabetes outcomes in comparison to existing insulins. Dr. Del Prato highlighted results from a recent study that compared Novo Nordisk’s weekly insulin (icodec) to Glargine U100 (Lantus) in people with type 2 diabetes:
-
Both insulins showed a similar reduction in A1c.
-
Icodec showed improved glucose profiles for self-monitored blood glucose (SMBG).
-
Rates of hypoglycemia were low for both insulins.
-
Weight gain, which is common when starting insulin, was the same for both insulins.
-
Icodec did not show any new safety issues.
Research is still to come on weekly basal insulin, but it looks promising.
Food as Medicine! Geisinger’s Fresh Food Farmacy
Michelle Passaretti (Geisinger Health System) presented data on the success of the Fresh Food Farmacy initiative. Fresh Food Farmacy was developed to meet the health needs of people with diabetes in Pennsylvania who do not have access to healthy foods (also known as being food insecure). diaTribe interviewed two leaders from Geisinger in 2018, Dr. Andrea Feinberg and Allison Hess; now, Fresh Food Farmacy has provided 482,219 total meals.
The data speaks to the power of food as medicine! The program participants had a:
-
2 percentage point reduction in A1C from a baseline of 9%
-
27% reduction in fasting glucose
-
13% reduction in cholesterol (including a 9.9% reduction in “bad” LDL cholesterol)
-
15% reduction in triglycerides
Fresh Food Farmacy also led to increased use of preventive care: flu shots increased by 23%, annual eye exams increased by 17%, and annual foot exams increased by 33%.
Compared to eligible individuals who did not participate, Fresh Food Farmacy participants saw:
-
49% lower hospital admissions rates
-
13% decrease in emergency department visits
-
27% more primary care visits
-
14% more endocrinologist visits
Participant surveys show significant improvements in quality of life, with 31% of people in the program rating their overall health as very good, compared to just 6% before participation. Additionally, 44% of Fresh Food Farmacy participants now rate their emotional and mental health as very good, compared to just 9% before the program. Passaretti emphasized that Fresh Food Farmacy is not a diet, but a lifestyle change, and that support for the individual’s entire household is necessary for success.








