Tandem’s Control-IQ Hybrid Closed Loop Algorithm Submitted to FDA
By Divya Gopisetty
 By Ruiyan Wang and Divya Gopisetty
By Ruiyan Wang and Divya Gopisetty
Control-IQ Hybrid Closed Loop on track to launch in the US by the end of 2019 for those ages 14 and older; first system with automatic correction boluses and no fingersticks CGM (Dexcom G6)
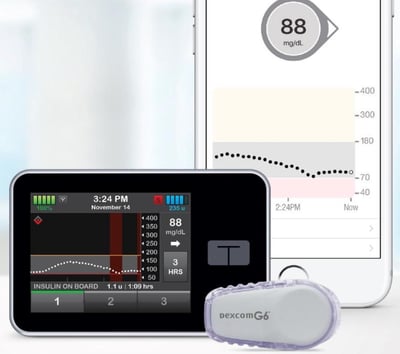
Tandem has submitted the algorithm for Control-IQ — an automated insulin delivery (AID) system — to the FDA. Control-IQ combines the t:slim X2 insulin pump, the Dexcom G6 CGM, and an algorithm built into the pump that adjusts basal insulin delivery and gives automatic correction boluses. If the FDA approves this, a US launch is expected at the end of 2019 for people ages 14 years and older. Because correctly dosing insulin is challenging, the automated features of Control-IQ are very appealing – the changes are designed to decrease the burden for people with diabetes and to improve blood sugar management. This system has been shown to reduce hypoglycemia (low blood sugar), increase time-in-range (70-180 mg/dl), and lower A1C.
.jpg) Tandem confirmed that Control-IQ will be offered as a free software update for: (i) in-warranty t:slim X2 pump users in the US; and (ii) those who purchase a Tandem pump in the US any time before December 31, 2020. The download will be offered through the Tandem Device Updater, which individuals access online from the convenience of their homes. Like the upgrade to add Basal-IQ, users will be able to access meaningful new innovation without needing a new pump – a big win!
Tandem confirmed that Control-IQ will be offered as a free software update for: (i) in-warranty t:slim X2 pump users in the US; and (ii) those who purchase a Tandem pump in the US any time before December 31, 2020. The download will be offered through the Tandem Device Updater, which individuals access online from the convenience of their homes. Like the upgrade to add Basal-IQ, users will be able to access meaningful new innovation without needing a new pump – a big win!
Control-IQ is designed to increase time-in-range (70-180 mg/dl) throughout the day and night. It will be the first hybrid closed loop system to:
-
Give automated boluses to correct for high blood sugars. In addition to adjusting basal rates to keep blood sugar in range, Control-IQ will give automated boluses up to once per hour during the day. If a user forgets to bolus, Control-IQ will “automate” and give approximately 60% of a full correction bolus with a target of 110 mg/dl. Users will still need to bolus insulin before mealtime.
-
Not require fingersticks from users for CGM calibration. Because Control-IQ is compatible with the Dexcom G6 CGM, no fingerstick calibrations are necessary.
This FDA submission follows the impressive results of the pivotal study of Control-IQ. Users on the system spent 2.6 more hours per day in-range (70-180 mg/dl) and lowered their A1C by 0.3% more than the group with the same pump and CGM without automation. Remarkably, all 168 participants completed the study. Control-IQ users spent 92% of the full six months with closed loop active. Control-IQ also achieved near-perfect scores on a technology acceptance questionnaire: ease of use was 4.7/5, usefulness was 4.6/5, trust was 4.5/5, and desire to continue using was an impressive 4.8/5 – an encouraging sign of the system’s simplicity (no modes to juggle), the no fingersticks G6, and limited alarms.
An additional pediatric study is recruiting to test the Control-IQ system to extend the age range down to six years. Tandem aims to get FDA approval for 6-13 years before next summer.
Control-IQ Algorithm Details
The Control-IQ hybrid closed loop algorithm aims is to keep users between 70-180 mg/dl as much as possible using a combination of automated basal insulin delivery and automated correction boluses. These decisions are based on readings from the Dexcom G6 CGM, which inform the algorithm’s decisions every five minutes. The algorithm includes the following components:
-
Automated Basal Rate Adjustment during the day (including after meals): the system targets a range of 112.5-160 mg/dl.
-
Automated Basal Rate Adjustment at night: the algorithm becomes more aggressive and tries to arrive at 112.5-120 mg/dl by morning.
-
Automated Bolus Corrections: these automated corrections can occur up to once an hour during the day, are triggered if the CGM is predicted to go over 180 mg/dl, and are calculated to give approximately 60% of a correction bolus with a target of 110 mg/dl. These boluses occur on top of the basal rate modulations described above. Users are still expected to meal bolus, but this feature should help reduce high blood sugars more quickly when a meal bolus is forgotten.
-
Exercise Mode: there is a button that the user can push if they wish that changes the target range to be closer to 140-160 mg/dl.
The algorithm requires all the normal pump settings: basal profile, insulin:carb ratio, insulin sensitivity factor; as we understand it, all those settings influence the system’s aggressiveness.







