As Glucagon Options Expand, Lilly Discontinues Emergency Kit
By Arvind Sommi
 Lilly has just announced that it will discontinue manufacturing its Glucagon Emergency Kits. Read why the company took this step and what your options are to access glucagon.
Lilly has just announced that it will discontinue manufacturing its Glucagon Emergency Kits. Read why the company took this step and what your options are to access glucagon.
The Glucagon Emergency Kit is an iconic piece of diabetes equipment that people with type 1 relied on for many decades. The kits themselves were a bright, ugly orange and included two separate vials and a long needle. They were not easy to use, and the people who did use them often felt nauseous afterward. But they kept many people alive and therefore will forever be cherished.
In light of this announcement from Lilly, we review why glucagon is an important part of your diabetes toolkit and the current options for next-generation emergency glucagon.
Why is glucagon important?
Hypoglycemia (glucose levels below 70 mg/dL) is a condition experienced by many people with diabetes. Since glucose provides energy to many organs throughout your body, hypoglycemia can have serious symptoms.
In some cases of hypoglycemia, your brain may have difficulty functioning. What begins as a headache and fatigue can quickly become severe with life-threatening symptoms, such as seizures and loss of consciousness. According to the CDC’s National Diabetes Statistics Report, in 2016 alone, 235,000 people went to the emergency room as a result of severe hypoglycemia.
Hypoglycemia exists on a spectrum – when your glucose level falls below 70 mg/dl, you might want to eat some carbohydrates or glucose tablets to raise it. However, these remedies take around 15 minutes to take effect as your body digests and absorbs the glucose.
In cases of severe hypoglycemia (glucose levels below 54 mg/dL), you may not have 15 minutes to spare. Moreover, consuming carbohydrates is not a viable option if you are unable to swallow or are unconscious. In this situation, another person has two options: call an ambulance or use glucagon.
Just as insulin is a hormone that lowers blood glucose, glucagon is a hormone that rapidly increases blood glucose. For many years, companies such as Lilly and Novo Nordisk have produced “Glucagon Emergency Kits” that contain a vial of glucagon and a syringe, as shown below.
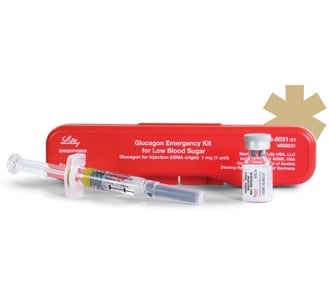
Why is Lilly discontinuing their Glucagon Emergency Kit?
Although Lilly’s Glucagon Emergency Kit was life saving, it requires preparation time and someone to administer the glucagon with a syringe – a potentially daunting task, especially for anyone unfamiliar with the procedure. Since 2019, easier next-generation emergency glucagon therapies have been approved:
-
Lilly’s Baqsimi is administered like a nasal spray and comes in a single-use dispenser. It does not require inhalation, meaning that it can be administered by another person even if the person with hypoglycemia is not able to follow instructions or is unconscious. It is a needle-free glucagon option and works as well as injectable glucagon.
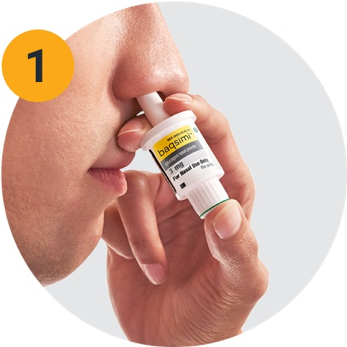
-
Gvoke HypoPen by Xeris, and Zegalogue by Zealand, are emergency glucagon autoinjectors with no visible needles. They require two simple steps: (1) remove the cap; and (2) press the pen against the skin (the person’s arm, leg, or wherever is available). Upon contact with the skin, the automatic injector will deliver a rescue dose of glucagon and retract the needle. Gvoke and Zegalogue also come in pre-filled syringes that are ready for manual injection as alternative options to the autoinjectors.
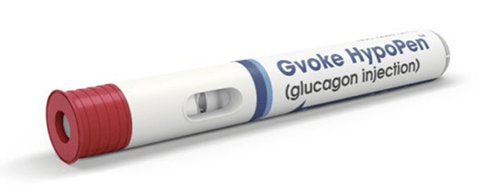
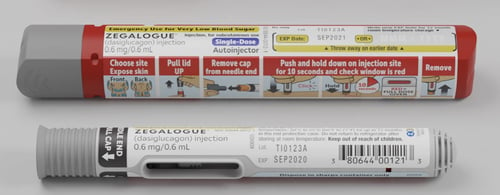
Lilly says that these new products have decreased demand for the older Emergency Glucagon Kit. In response, Lilly announced it will discontinue production of the Emergency Glucagon Kit on December 31, 2022. For those who prefer the older kit, Amphastar Pharmaceuticals produces a generic version with no plans to remove it from the market. Additionally, Novo Nordisk makes a similar GlucaGen HypoKit that requires the same time and preparation steps as the Lilly kit.
Sarah Noel, senior director of US Diabetes Advocacy & Professional Relations at Lilly, said, “By announcing Lilly’s Glucagon Emergency Kit discontinuation well in advance of the last manufacturing date, we can help people who have traditionally relied on our Glucagon Emergency Kit to prepare and discuss alternative glucagon options with their healthcare providers.”
For more information about emergency glucagon, read this article from one of our Musings events, “Taking the Fear out of Low Blood Sugar: Next-Generation Glucagon.”








