Diabetes puts you at risk of liver disease. Learn about the relationship between type 2 diabetes and non-alcoholic fatty liver disease (NAFLD), complications like NASH...
Guidelines
215 readers recommended
Manage diabetes better with the latest ADA standards for glucose, cholesterol, and blood pressure. Discuss your targets with your healthcare team for personalized care.
92 readers recommended
More than 50 percent of people with diabetes are at risk for dry eyes, which can harm vision if untreated. Recognize symptoms like redness and stinging. Seek treatment...
87 readers recommended
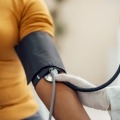
People with diabetes risk complications like stroke and heart attack. The ADA's 2023 care standards, along with regular exams and a healthy lifestyle, can reduce these...
101 readers recommended
People with diabetes risk eye issues like retinopathy and glaucoma. Following the ADA's 2023 care standards and regular eye checks can mitigate these risks.
48 readers recommended
Uncover the complex link between obesity, asthma, and diabetes, plus effective management strategies to deal with allergens during peak allergy season.
296 readers recommended
Explore diabetes-friendly fast food options. Get dietitian-approved picks and tips for healthier choices while maintaining your carb budget.
129 readers recommended
Lifestyle changes like diet and exercise are key to the prevention and management of diabetes-related complications like foot disease. Here’s why it’s important to be...
42 readers recommended
Research shows screening children ages 2-6 best predicts childhood type 1 diabetes. Early screening also leads to more timely treatment, better outcomes, and reduced...
170 readers recommended
The debate over how many carbohydrates people should consume has raged for decades. Experts share their perspectives on low, very low, and moderate carb diets for ideal...
40 readers recommended
Obesity management options include lifestyle changes, medication, and surgery. Here's what you need to know to make informed decisions about what treatments are optimal...
63 readers recommended
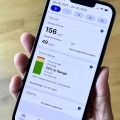
A lot has happened this year advancing time in range as a primary glucose metric in diabetes care globally. Though we aren’t there yet, a panel shared their thoughts on...
56 readers recommended
A panel shared insights at the ADA’s 83rd Scientific Sessions on incorporating hypoglycemia as a quality measure in diabetes. Each expert noted the importance of...
76 readers recommended
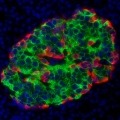
On the search for a type 1 diabetes cure, scientists are experimenting with how to engineer beta cells to survive immune system attacks. At the American Diabetes...
53 readers recommended
Figuring out when and how to exercise can be one of the most difficult parts of living with diabetes. At the ADA's 83rd Scientific Sessions, experts shared strategies...
48 readers recommended
Early screening for type 1 diabetes is vital for timely treatment and prevention of certain complications. With recent advancements such as the FDA approval of Tzield (...
446 readers recommended
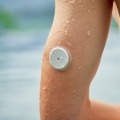
Medications like aspirin, Tylenol, and vitamin C can potentially impact the accuracy of continuous glucose monitors (CGMs). Here are steps to ensure reliable diabetes...
63 readers recommended
Maximize fun in the sun this summer with our skincare guide for people with diabetes. Here we share tips for how to protect your skin and stay healthy during the hottest...
73 readers recommended
FDA warns people to avoid using some forms of compounded substitutes of semaglutides that lack the safety and efficacy of popular weight loss drugs Wegovy, Ozempic, and...
28 readers recommended
The National Institute of Clinical Excellence recently announced it was the first to expand access to automated glucose monitors for children with type 2 diabetes. The...