Vertex Releases New Data on Potential Cure for Type 1 Diabetes

Key takeaways:
- Zimislecel (VX-880) is an investigational islet cell therapy by Vertex that shows promise as a functional cure for type 1 diabetes.
- A study presented in June 2025 showed that, after one year, stem cell islet transplants provided the first-ever sustained insulin production in people with type 1 diabetes.
- The zimislecel treatment requires ongoing immunosuppressive therapy.
Study results presented in June 2025 showed that stem cell islet transplants provided the first-ever sustained insulin production in a group of people with type 1 diabetes.
In an ongoing clinical trial presented at the American Diabetes Association (ADA) annual conference in Chicago, 12 participants who received a full dose of the Vertex therapy called zimislecel (VX-880) no longer needed daily insulin after one year, though all required ongoing immunosuppressive therapy.
Ten participants saw significantly improved glucose levels, spending more than 90% of the time in range. Two other participants showed signs of insulin production and spent more than 70% of the time in range, but still required some insulin.
The results showed that stem cell islet transplants provided the first-ever sustained insulin production in a group of people with type 1 diabetes.
Before receiving the treatment, all participants had hypoglycemia unawareness and experienced multiple severe low blood sugar events in the previous year. After treatment, none of these events were reported, and no serious side effects were reported.
What is zimislecel therapy?
A type of experimental islet cell therapy, zimislecel aims to restore natural insulin production in people with type 1 diabetes. This would result in a drastic improvement in quality of life as those with the disease would no longer require insulin or experience severe, sometimes life-threatening, episodes of hypoglycemia.
Cell therapies like zimislecel work by transplanting healthy insulin-producing cells into the body. If successful, the new cells would replace non-functioning ones and ultimately restore insulin production.
Similar to people who receive organ transplants, those treated with zimislecel must take immunosuppressants to prevent the immune system from rejecting new beta cells.
Because of the risk of infection associated with immunosuppressants, only those with severe hypoglycemia unawareness have been eligible for Vertex’s trial. This is because these candidates have the most to gain from this type of therapy, given the risks associated with hypoglycemia unawareness.
The history behind Zimislecel
Vertex first announced the development of zimislecel in 2021. Data from the first two participants in the trial were presented by Dr. James Markmann, chief of the division of transplant surgery at Massachusetts General Hospital, at the 2022 American Diabetes Association (ADA) conference.
In the first phase of the trial, each participant was given half the dose of beta cells estimated to be required. A common practice in these types of studies is starting with half the expected dose and increasing it as the trial progresses.
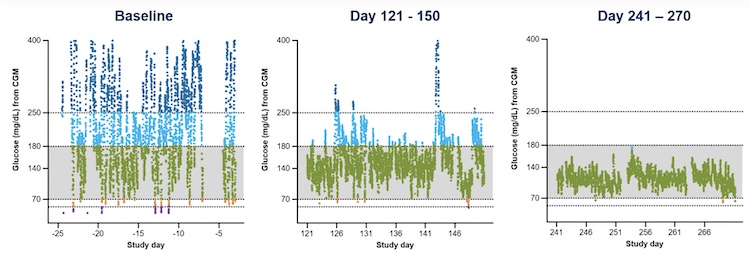
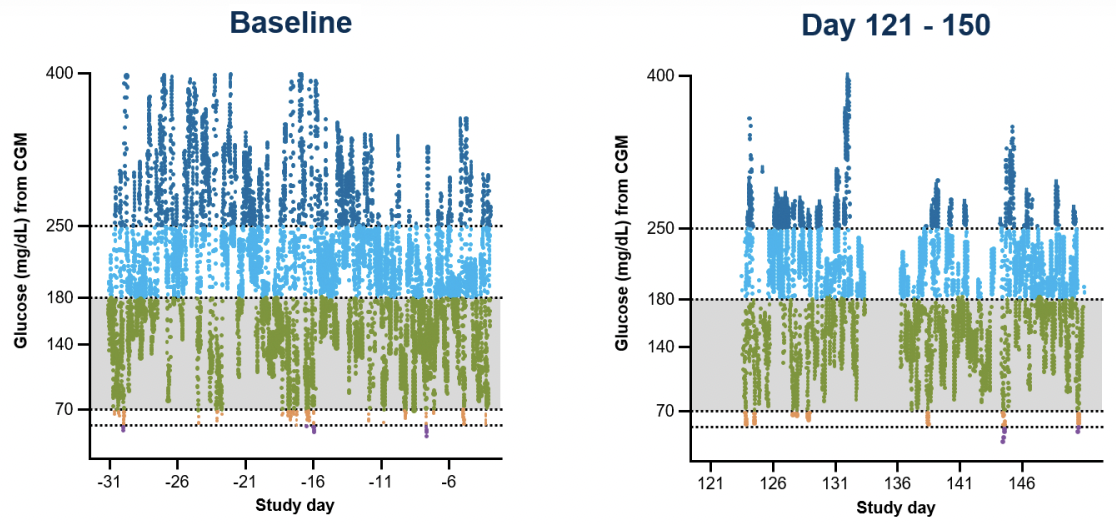
The following results show astonishing improvements in A1C, time in range, and reduced insulin needs for each participant. For reference, the ADA Standards of Care recommends a target time in range of 70% (70-180 mg/dL). There is no “standard” insulin dose, as daily insulin doses are determined by a healthcare provider based on the needs of each individual. To put this in perspective, here’s a closer look at some of the data from two participants.
Participant 1
| Study day | Day 0 (baseline) | Day 121-150 | Day 241-270 |
| Daily Insulin dose | 34 units | 2.6 units | 0 units |
| Time in Range | 40.1% | 81.4% | 99.9% |
| A1C | 8.6% | 6.7% | 5.2% |
Participant 2
| Study day | Day 0 (baseline) | Day 121-150 |
| Daily Insulin dose | 25.9 units | 18.2 units |
| Time in Range | 35.9% | 51.9% |
| A1C | 7.5% | 7.1% |
Participant deaths: what happened?
Despite positive results in the early stages of the zimislecel trial, Vertex halted the study in January 2024 after two participants died. Neither death is believed to be related to zimislecel.
One participant died of meningitis after a sinus surgery unrelated to the study. Reichman explained that the participant had been given a high dose of steroids before and after the procedure (steroids are toxic to islets, aka insulin-producing cells in the pancreas). The participant was also on immunosuppressants, which is necessary following zimislecel, but since high-dose steroids also lower the immune system, it could have left them vulnerable to infection.
The other participant died from a traumatic brain injury following a motorcycle accident caused by a severe episode of hypoglycemia.
One participant remains unknown, but the other, Brian Shelton, 66, was said to be the first person “cured” of type 1 diabetes with Zimislecel. Shelton successfully started producing his own insulin in 2021 six months after the investigational treatment.
The future of Zimislecel
While there was initial concern about the two participant deaths being related to the procedure, Reichman said that no severe adverse events were related to the islet cells themselves.
So far, the safety profile of zimislecel is similar to what would be expected given the use of immunosuppressants, the surgical procedure to implant the cells, and the participants’ medical history.
Though early, the results after a year represent a potential breakthrough toward a functional cure. Larger, longer-term studies are planned to evaluate the safety and durability of the approach. A current trial underway is expected to complete enrollment of 50 participants by summer 2025.
When will this become available to more people with type 1 diabetes?
Unlike other interventions, such as pancreas transplants or beta cell islet transplants from human donors, zimislecel has the potential to be used on a much larger scale.
“Right now in the U.S., there are only about 1,000 pancreas transplants available, so supply is a significant problem,” Markmann said. “One of the most important aspects of this work is that there can be an unlimited supply of beta cells for transplantation going forward.”
After all of the studies are completed, Vertex needs to submit an application to the FDA before it can be approved for use. Encouraged by recent results, the company has accelerated its timeline for FDA submission from 2030 to 2026. With the FDA fast-track designation, zimislecel could become available as early as 2027.
“This is groundbreaking work and a real leap forward for the field,” said Markmann. “While we cannot say exactly when this will become available, we are much closer to a functional cure for type 1 diabetes than we were before this approach.”
Learn more about treatments and the search for a type 1 diabetes cure here:
- A Promising Step Towards Insulin Independence in Type 1 Diabetes
- Trial Watch: VX-264 For Type 1 Diabetes Discontinued
- FDA Approves Lantidra, the First Ever Cell Therapy for Type 1 Diabetes