What’s Coming and What’s Delayed in Continuous Glucose Monitoring?

By Albert Cai
Updates and delays from Abbott, Dexcom, Medtronic, and Senseonics
With several clinical trials on hold due to the COVID-19 pandemic, we’re bringing you a roundup of the latest updates on future continuous glucose monitors (CGM). Understandably, the FDA also announced a few months ago that it would focus its efforts on devices related to COVID-19. With the disclaimer that it’s impossible to know exactly when the pandemic will subside, when trials might resume, and how FDA reviews might be affected, here is the latest news we’ve heard from companies.
Click to jump to a product, which are organized alphabetically.
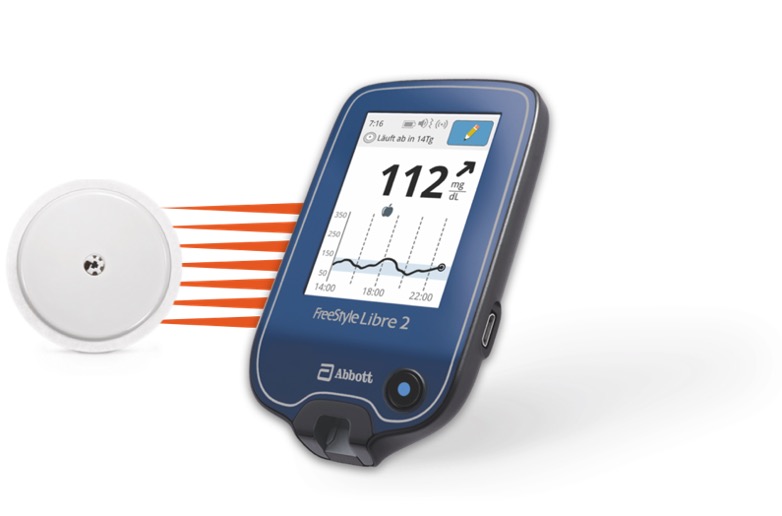
What’s new? FreeStyle Libre 2 keeps the same “scanning” feature as the original FreeStyle Libre, but adds Bluetooth connectivity. This is important because it enables optional high and low glucose alerts. Users who enable these alerts will be able to get a notification on their reader or phone whenever their glucose reading goes above or below their specified ranges. Looking ahead, the Bluetooth feature will also allow FreeStyle Libre 2 to be part of automated insulin delivery systems (AID), like Insulet’s Omnipod Horizon.
Like the original FreeStyle Libre, FreeStyle Libre 2 has 14-day wear, is factory-calibrated (no fingerstick calibrations required), and can be scanned with either a phone or a reader device (the reader for FreeStyle Libre 2 is blue, instead of black). Importantly, FreeStyle Libre 2 will be offered at the same price as the original FreeStyle Libre.
When’s it coming? The FreeStyle Libre 2 has already launched in a few European countries (we know of Germany and Norway) and will launch in others soon. In the US, FreeStyle Libre 2 has been under FDA review for over a year. In March, Abbott said that it was working through “some finishing items” and was “very confident” the device would be cleared soon.
What’s new? Dexcom’s G7 will be fully disposable (the transmitter and sensor are combined and thrown away together) and have longer wear (we believe somewhere around 14-16 days). Remember that the Dexcom G6 sensor lasts for 10 days but has a transmitter that is re-used for 90 days. The G7 will be considerably slimmer than G6 and will have a lower cost of manufacturing in bulk, though consumer pricing is not yet determined – we imagine it will be similar. The G7 will keep the same accuracy, no fingerstick calibrations, and Bluetooth connectivity as the G6.
Dexcom has been developing G7 in partnership with Verily, the division of Alphabet formerly known as Google Life Sciences. There has been mention from Verily that an accelerometer may also be built-in to the G7 device, but we aren’t sure if that feature made it into the final version of G7. Having a built-in accelerometer could allow the G7 to also track physical activity, like a Fitbit or other fitness tracker.
When’s it coming? Dexcom planned on launching G7 in “early 2021,” but with most clinics placing new trials on hold, Dexcom is expecting a “minimum delay of approximately six months.” It’s difficult to know when clinics will be able to conduct trials (and when people will feel comfortable enrolling in trials), but assuming a six-month delay, G7 could be on the US market sometime in the second half of 2021.
What’s new? Medtronic’s next CGM, referred to as “Project Zeus,” will reduce the number of required fingerstick calibrations and have improved accuracy (compared to its current offering, Guardian Sensor 3). The new CGM will require day-one calibration (unclear on the number of fingersticks that will be required on day one), compared to Guardian Sensor 3, which requires at least two fingerstick calibrations every day. Medtronic expects Project Zeus to launch with a “non-adjunctive” indication, meaning users will be able to bolus insulin based on CGM reading alone, and not have to perform a confirmatory fingerstick. the new CGM will keep the same seven-day wear, size and shape, and reusable transmitter component as the Guardian Sensor 3 (pictured above).
When’s it coming? The trial for Project Zeus began in June 2019 and is expected to wrap up within the next month. Medtronic expects to submit the CGM to the FDA by the “end of the summer."
 Senseonics Eversense XL (180-day)
Senseonics Eversense XL (180-day)
What’s new? The “XL” extended life-version of Senseonics’ Eversense in the U.S. will have the same size and features as the original Eversense, but the Eversense XL is implanted for 180 days, rather than the 90-day Eversense. As a reminder, the Eversense sensor is implanted in the users’ upper arm in a clinic and remains there for the sensor duration; a silver-dollar sized on-body transmitter is worn on the outside of the arm to deliver readings to a smartphone. Senseonics is targeting reducing calibrations from 2 per day to 1 per day with same non-adjunctive indication.
When’s it coming? Eversense XL is already available in Senseonics’ European markets. The trial for Eversense XL in the US wrapped up in late March, and Senseonics has previously aimed for FDA clearance in “late” 2020. We aren’t sure whether that timeline has been pushed back due to COVID-19, but the fact that the trial has already completed is encouraging.