Impressive Results From the iLet Bionic Pancreas Pivotal Trial
By Matthew Garza
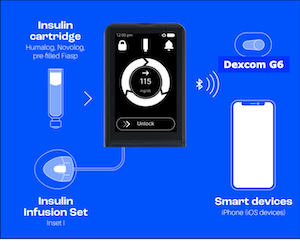 The iLet Bionic Pancreas is currently the closest automated insulin delivery system to a fully closed loop system. At ADA's 82nd Scientific Sessions, a panel of experts shared the impressive results from the recent iLet Bionic Pancreas pivotal trial.
The iLet Bionic Pancreas is currently the closest automated insulin delivery system to a fully closed loop system. At ADA's 82nd Scientific Sessions, a panel of experts shared the impressive results from the recent iLet Bionic Pancreas pivotal trial.
On the first day of the American Diabetes Association’s 82nd Scientific Sessions, an impressive lineup of presenters delivered extensive results from Beta Bionics’s iLet Bionic Pancreas pivotal trial. The iLet Bionic Pancreas is currently the closest automated insulin delivery (AID) system to an FDA-approved fully closed loop system – requiring minimal input from the user and no carb counting.
What is the iLet Bionic Pancreas?
The iLet Bionic Pancreas system uses a tubed insulin pump that is roughly the size of a credit card and which houses the AID algorithm, a Dexcom G6, and a smartphone or other device. It is worn similar to other AID devices, with the pump attaching on the abdomen and the Dexcom G6 being worn on the back of the upper arm or on the abdomen.
One of the things that makes the iLet Bionic Pancreas so unique is the minimal input required from the user. All that is needed is the user's weight when they first initiate the device and then adjustments for glucose target (usual, lower, or higher) and the meal type (breakfast, lunch, or dinner) and size (usual for me, less, or more). All insulin dosing is then determined by the device's algorithm using this information.
The pivotal trial is part of the data being submitted to the FDA to try and obtain clearance for the device to make it available for people with diabetes. As of right now, the system is only available for investigational use (like in a clinical trial).
What did the pivotal trial for the iLet Bionic Pancreas show?
Dr. Gregory Forlenza, associate professor of Pediatrics at Barbara Davis Center for Diabetes at the University of Colorado, began the presentation by outlining the design of this pivotal trial. There were 440 participants included (165 children and teens and 275 adults). The trial was relatively diverse, with almost 1 in 4 participants identifying as non-white. At the start of the trial, 88% of the participants were already using a continuous glucose monitor (CGM), and 31% were already on a hybrid closed-loop AID system.
The participants were randomly assigned into one of three different treatment groups:
-
Those who continued their standard insulin delivery method (whatever technology they were using at the start) with a Dexcom G6 CGM – that included both children and adults
-
Those using the bionic pancreas with Humalog and Novolog insulin – that also included both children and adults
-
Those using the bionic pancreas with Fiasp insulin – that had adults only
Following Forlenza’s description of the design, Davida Kruger (nurse practitioner at Henry Ford Health System in Detroit, Division of Endocrinology, Diabetes, and Bone Disorders), Dr. Laurel Messer (assistant professor, Pediatrics-Barbara Davis Center), and Dr. Steven Russell (associate professor of medicine, Harvard Medical School and Massachusetts General Hospital Diabetes Research Center) delivered data from three subsections of the trial.
Compared to those using their standard insulin delivery method, adults using the bionic pancreas with Humalog and Novolog saw their A1C decrease by an average of 0.5 percentage points (compared to 0.1 percentage points in the control group) at 13 weeks. A closer look at data also showed that 43% of those using the bionic pancreas with Humalog/Novolog saw their A1C decrease by more than 0.5 percentage points and 23% saw it decrease by more than 1.0 percentage points. In addition, Time in Range (TIR) improvements (on average there was an 11 percentage point increase in TIR) were observed after only one day.
In the adult group that was using the bionic pancreas with Fiasp, results were almost identical to the other adult group using the bionic pancreas with Humalog and Novolog. Russell highlighted that one of the only major differences was a minor increase in the number of participants achieving a Time in Range greater than 70% (58% of participants in the bionic pancreas with Fiasp group and only 47% in the bionic pancreas with Humalog/Novolog group).
The youth participants saw similar results. Those using the bionic pancreas achieved an average A1C reduction of 0.5 percentage points compared to no improvement in the standard care group. And again, similar to the adults, 51% of those using the bionic pancreas saw their A1C decrease by more than 0.5 percentage points and 29% saw it decrease by more than 1.0 percentage points. In addition, Time in Range (TIR) improvements (on average there was an 10 percentage point increase in TIR) were observed almost immediately.
“In children who are going to have a lifetime of diabetes, this is significant in reducing morbidity and mortality,” said Messer.
In adults and in the youth group, there was a greater improvement in those who started the trial at a higher A1C, and across almost all demographic breakdowns and insulin delivery systems the Bionic Pancreas outperformed the standard care group in reducing A1C. There were minimal severe adverse events in all groups – with only a few cases of severe hypoglycemia.
.jpg)
Demographic breakdown of A1C reductions in adult group using bionic pancreas with Humalog/Novolog. Some of the most impressive data from this graph includes an average 1.1 percentage point reduction in A1C for people who are non-white and non-Hispanic, a 0.9 percentage point reduction in A1C for those who have less than a Bachelor’s degree, and a 0.7 percentage point reduction in those who make less than $100K a year.
.jpg)
Initial Insulin Delivery breakdown of A1C reductions in the adult group using bionic pancreas with Humalog/Novolog. For those who were initially on MDI, pump with no automation, or hybrid closed-loop and then switched to the bionic pancreas, there were significant reductions in A1C across the board – the greatest being an average of 1.0 percentage point drop in those who were initially on MDI.
.jpg)
Initial Insulin Delivery breakdown of A1C reductions in the youth group using bionic pancreas with Humalog/Novolog. For those who were initially on MDI, pump with no automation, or hybrid closed-loop and then switched to the bionic pancreas, there were significant reductions in A1C across the board – the greatest being an average of 1.0 percentage point drop in those who were initially on MDI.
These results are extremely impressive. The bionic pancreas showed significant improvements in A1C and Time in Range even when compared to people who were already on a hybrid closed-loop AID system such as Control-IQ or MiniMed 670G. It also included a relatively diverse group of participants across a wide age range and insulin delivery distribution – resembling more closely the general population than many other comparative AID trials.
Is this the only device that Beta Bionics is working on?
Beta Bionics is also currently testing the Bihormonal iLet Bionic Pancreas AID system to help people manage their diabetes. This system both monitors blood glucose levels and administers insulin and glucagon – a hormone from the pancreas that raises blood sugar levels. The hope with the dual hormones is that glucagon can be delivered during times when glucose levels are low in order to stabilize them without user interaction, eliminating the need for carbs.
You can read more about these results in our preliminary coverage of this trial from the ATTD 2022 conference.








