Can’t Find the Remote Control? No Problem – New Insulin Pump from Calibra Goes the Distance Alone
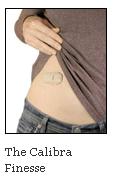
In our view, the main advantages of the Finesse are its simplicity (once the user is familiar with the device, insulin can be easily bolused through clothing by clicking the buttons - dosing seems like it would be much quicker overall as well), its discretion (the company assured us the device can be worn under most clothing without being visible), and small size (Calibra reported the device to be in the ballpark of 2” long, 1” wide, and ¼” thick - very small compared to the other pumps currently on the market). Because the device is only intended to administer bolus insulin and not basal insulin, users still need to take their daily dose of Lantus or Levemir. And, it will not be an option at insulin initiation or for type 2 users only on basal therapy - but, it will be a technology that could dramatically help those who should be moving to basal-bolus but are avoiding it. To be sure, we believe this patch-pen could be a simpler alternative for patients transitioning from basal therapy to MDI or a more convenient way for patients already on MDI to administer mealtime and correction insulin. We also think patients who have avoided pumps to date due to size might very much like Calibra's look and feel. We look for payors to set reimbursement plans shortly, though we believe Calibra would do a great deal of good related to better adherence of insulin. As we understand it, Calibra is planning to launch the Finesse sometime in 2010. --JS







