The FDA Approves Medtronic’s MiniMed 670G Hybrid Closed Loop System

By Ava Runge and Adam Brown
Coming to US in Spring 2017: What it is, what it isn’t, and how to get it
Want more news like this? Sign Up Now!
In earlier-than-expected and very good news for people with diabetes, the Food and Drug Administration (FDA) approved Medtronic’s MiniMed 670G hybrid closed loop insulin pump and more accurate Guardian continuous glucose monitoring (CGM) sensor, Guardian Sensor 3.
This news represents a major milestone for diabetes technology a little over a decade after JDRF launched the “artificial pancreas” project in 2005. It can’t be emphasized enough how exciting this development is for anyone who takes insulin and has access, since it’s definitely one step closer to the long-term goal of the artificial pancreas – the “ultimate” closed loop.
Based on study results, the 670G will reduce time at dangerous high and low blood sugar levels, improve time-in-range, reduce glucose variability, bring much greater nighttime safety and target morning blood sugars, and reduce diabetes hassle. The 670G is not a “cure” and still requires some user effort (see below), but it is a very welcome advance that will make insulin therapy safer and easier for many people with diabetes – and potentially greatly improve their control.
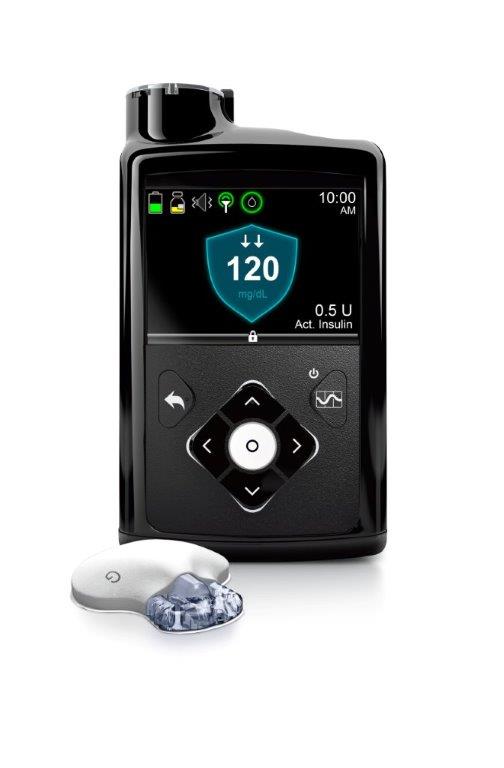
The 670G will launch in spring 2017 in the US, bringing the first system to automate basal insulin delivery to market – increasing insulin delivery when glucose is going high and decreasing insulin delivery when glucose is going low. Users still have to bolus for meals, but the system automates basal insulin delivery and targets a set blood glucose of 120 mg/dl, which is particularly effective for improving overnight blood sugars and reducing lows.
The MiniMed 670G is FDA approved for people with type 1 diabetes ages 14 and over, though a pediatric study (7-13 year olds) is currently underway to investigate an expansion in how it can be prescribed. The device cannot be used in children less than seven years old and those on less than eight units of insulin per day.
The system was granted priority review following the June submission, and the FDA worked quickly to approve it in just 104 days – about one third of the typical time for new devices like this.
International approval of the MiniMed 670G is expected in summer 2017.
Medtronic will offer this pump at similar pricing to its current pump and CGM offerings. A Priority Access Program will allow users to go from the new MiniMed 630G system with low glucose suspend only (stops insulin at low glucose) to the 670G hybrid closed loop for $0 or $299. Don’t have the MiniMed 630G? At this point, it is not clear if current Medtronic pumpers on Revel or 530G pumps will be able to upgrade straight to the 670G (see below). Inquire directly with Medtronic by calling 1-800-646-4633.
Many companies are working on similar automated insulin delivery products – Animas, Tandem, Insulet, Bigfoot, and others – meaning several options should exist for people with diabetes in the coming years. A special mention goes to JDRF and the tremendous vision and commitment of Jeffrey Brewer and Dr. Aaron Kowalski for pioneering this approach back in the early 2000s.
Read on for cost details, how to upgrade, and answers to frequently asked questions (click to jump directly to an answer).
Frequently Asked Questions
How It Works
Launch and Upgrade Details
-
The 670G is approved now, so why is launch not until spring 2017?
-
How can I get on the 670G if I’m already using a Medtronic pump?
-
Can I get the 670G if I am currently using a non-Medtronic pump (Animas, Insulet, Tandem, Roche)?
CGM and Connectivity Questions
-
Is the new Guardian CGM sensor better than the Medtronic Enlite?
-
In terms of safety, what will happen if my sensor fails or is inaccurate while I’m wearing the 670G?
-
Does the MiniMed 670G work with MiniMed Connect, allowing remote monitoring of pump and CGM values?
Other Questions
Answers
How It Works
The MiniMed 670G consists of an insulin pump (with tubing), a continuous glucose monitoring (CGM) sensor inserted under the skin, and a transmitter worn on the body. When the 670G is operating in “Auto Mode,” it receives a glucose value from the CGM every five minutes and uses it to automatically adjust basal insulin delivery, targeting a level of 120 mg/dl. If glucose starts trending high, the system may deliver more basal insulin to prevent/limit high blood sugars. If glucose starts going low, it may deliver less basal insulin to prevent/limit hypoglycemia. The target can be temporarily raised to 150 mg/dl during activity, and the algorithm adapts over time as conditions change and it learns patterns – for instance, it may deliver more insulin during times of sickness, or less insulin during a week of high activity.
Unlike many academic systems that run the closed-loop control algorithm on a smartphone, the 670G algorithm is fully integrated within the pump itself. Users only need to wear the Guardian CGM sensor and the MiniMed 670G pump – no need to carry a separate CGM receiver or phone.
What is a “hybrid closed loop?” Does it take care of everything? What if I miss a meal or correction bolus?
The 670G is considered a “hybrid closed loop” system because it is not fully automated – it still requires manual food and correction insulin boluses, as it only automates basal insulin.
Still, this means the 670G will take care of insulin dosing in the background (on top of boluses), which is particularly valuable at night (about 80%-plus time-in-range from study data), and can help mitigate many highs and lows during the day. Food boluses will also benefit from an automated system that works between meals. For example, if a user took too much or too little insulin for a meal, the system might reduce or increase insulin delivery to keep glucose in range.
It’s important to note that the MiniMed 670G will NOT give automatic correction boluses based on high CGM readings. This means that if a meal bolus is completely missed, glucose will still go high. The 670G will increase basal insulin in response to the high – up to a certain point – to bring blood sugar down, but it won’t give a large automatic correction bolus at one time on its own (future systems will add that). The system will alert the user to a significant high glucose number, ask for a fingerstick, and then prompt a manual correction bolus.
Is there evidence that the 670G improves blood sugars? What did the pivotal trial show?
The pivotal study presented this summer suggested that this hybrid closed loop approach significantly improves blood sugars and safety. Adolescents and adults spending three months on the MiniMed 670G saw:
-
A 0.5% reduction in A1c, bringing participants from a low initial A1c of 7.4% to 6.9%;
-
44% less time spent with low blood glucose (under 70 mg/dl);
-
11% less time spent over 180 mg/dl and an 8% improvement in time-in-range (71-180 mg/dl);
-
And much better overnight glucose levels.
Of the 124 people in the trial, a striking 80% opted to continue using the device through the FDA’s continued access program – encouraging enthusiasm for the system – plus FDA comfort with real-world use of the device. Read more on the study findings here, read our interview with a trial participant here, and listen to JDRF’s call to hear one person’s experience.
Launch and Upgrade Details
The 670G is approved now, so why is launch not until spring 2017?
Medtronic needs time to organize manufacturing, insurance coverage, and appropriate training of healthcare providers, employees, and 670G users. The company is proceeding cautiously with the launch, given the importance of this new product and of managing expectations.
How can I get on the 670G if I’m already using a Medtronic pump?
Medtronic is offering a Priority Access Program for users to go from the new MiniMed 630G system to the 670G hybrid closed loop for $0-$299. These individuals will be offered first-in-line access to the 670G. Read more here.
Don’t have the MiniMed 630G? At this point, it is not clear if current Medtronic pumpers on Revel or 530G pumps will be able to upgrade straight to the 670G. One option is to upgrade to the 630G now and then take advantage of the 670G Priority Access Program. Upgrading from an older Medtronic pump to the MiniMed 630G, however, is expensive: $599 or $3,100 out of pocket (including a $400 trade-in credit, depending on whether the old pump was purchased after or before May 1, 2016).
Medtronic Diabetes can be contacted for more details.
Can I get the 670G if I am currently using a non-Medtronic pump (Animas, Insulet, Tandem, Roche)?
It has not yet been disclosed if Medtronic will offer a discount program for users on other pumps to switch over to the MiniMed 670G.
Pumps have a four-year warranty before insurance will typically cover a new one. That means pumps acquired in the past four years will probably require users wait until the warranty expires before insurance will cover the MiniMed 670G. A possible exception may allow people who change insurance to get the 670G covered through the new plan.
The complete MiniMed 670G system – pump, CGM transmitter, and paired glucose meter – will be priced similarly to Medtronic’s current CGM-integrated pumps at approximately $7,899. We don’t yet know if Medtronic will offer a discount for those without insurance or those desiring to pay cash.

CGM and Connectivity Questions
Do I still have to insert infusion sets and sensors?
Yes. Similar to other CGMs and pumps, the 670G requires an infusion set change every two to three days, a new sensor insertion every seven days, and at least two fingerstick calibrations per day (though four are recommended).
Is the new Guardian CGM sensor better than the Medtronic Enlite?
Yes, the Guardian sensor is much more accurate than Medtronic’s current sensor in the US, Enlite. Guardian’s average error relative to lab measurement is 10.6%, when providing a minimum of two fingerstick (glucose meter) calibrations per day. The FDA actually recommends four fingerstick calibrations per day, which improves the error to 9.6%.
This accuracy is slightly worse than the Dexcom’s G5 average area around 9%, with two calibrations per day, but much better than the previous Enlite sensor’s approximately 14% error. Abbott’s FreeStyle Libre “consumer” version (real-time, under FDA review) has an average error at about 11%, but it does not require any fingerstick calibrations.
Aside from the automatic basal insulin delivery, the Guardian CGM is only approved for use alongside, and not in replace of, fingerstick glucose meters. 670G users are still supposed to take a confirmatory fingerstick before taking a bolus.
In terms of safety, what will happen if my sensor fails or is inaccurate while I’m wearing the 670G?
The system will revert to open loop (“normal” pumping) using pre-programmed basal rates.
Does the MiniMed 670G work with MiniMed Connect, allowing remote monitoring of pump and CGM values?
No, it is not compatible with the MiniMed Connect device. Those desiring or using remote monitoring must use the MiniMed 530G. Medtronic has future plans to build Bluetooth connectivity into its pumps, but there is no current timeline on when these might launch.
Other Questions
Can I use the 670G if I have type 2 diabetes and am on insulin therapy?
While the 670G is currently only approved for people with type 1 diabetes over 14 years of age, labeling could expand over time to include younger people with type 1 and people with type 2 diabetes. Providers can technically prescribe products off-label, but insurance may be difficult.
What’s coming up next from Medtronic?
Medtronic has begun a limited launch of its MiniMed Pro Infusion set with BD FlowSmart technology – you can order it from the online store here. The set has a number of improvements, including a cannula with two holes from which insulin can flow (preventing blockages and flow interruptions).
Sugar.IQ, the new app from Medtronic and IBM Watson (check out a commercial here), is currently available to a small test group of 100 MiniMed Connect users. It will launch more broadly later this year – we are very excited! The app identifies patterns based on retrospective CGM and pump data – “[At 7 AM] Planning your day? I see you tend to go low on Saturdays between 12 PM and 3 PM.” Sugar.IQ also includes a food diary, enabling users to log meals and receive actionable insights – “Having Tuna salad? Be aware that you tend to go low when you eat this meal.”
Medtronic’s new Guardian sensor is also under FDA review as a standalone, mobile CGM – sending data straight from the on-body transmitter to a smartphone app via Bluetooth (no pump needed). Launch is expected in the US by April 2017.
[Photo Credit: Medtronic]
Want to receive more diaTribe articles like this?
diaTribe is a free online publication run by The diaTribe Foundation. Our mission is to improve the lives of people with diabetes by empowering them with useful, actionable information that gives them hope for a better future and helps them live happier, healthier lives.