FDA Approves Senseonics’ Eversense 90-Day Implantable CGM, On-Body Transmitter, and Smartphone Apps
By Jimmy McDermott
 By Jimmy McDermott, Brian Levine, and Adam Brown
By Jimmy McDermott, Brian Levine, and Adam Brown
First implantable CGM available in the US! Eversense is expected to launch July 2018
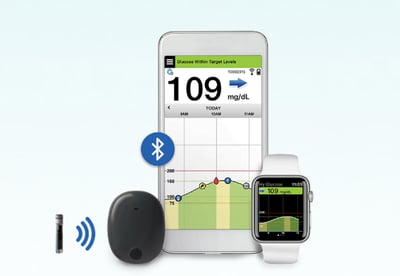
The FDA has approved Senseonics’ Eversense continuous glucose monitoring (CGM) system, the first implantable CGM in the United States. The system includes a pill-sized sensor implanted in the upper arm for 90 days, an on-body transmitter, and a smartphone app for viewing data (Apple, Android). Eversense’s 90-day wear removes the need for frequent sensor insertions required by other CGMs, which currently last for 7-14 days (e.g., Abbott’s FreeStyle Libre, Dexcom G5/G6, Medtronic’s Guardian Sensor 3).
The FDA has approved Eversense for users 18 years and older, and the first US insertions are expected this month. Eversense is very accurate, but it does requires two fingerstick calibrations per day, unlike the no-calibration Dexcom G6 and Abbott FreeStyle Libre; it is not approved for insulin dosing.
We have heard many positive things from Eversense users in Europe:
-
The insertion and removal procedure is quick – it takes five minutes in a healthcare provider’s office, leaving only a small cut. Local anesthetic (lidocaine) is used.
-
The Eversense transmitter is worn right above the sensor and uses a gentle adhesive – a win for those with allergies to other CGMs’ adhesive patches. The transmitter can be easily removed and put back on, leaving the implanted sensor in place. This also means users don’t have to worry about accidentally tearing out their sensor, since it is separate from the wireless on-body transmitter.
-
 This is the first CGM to provide on-body vibration alerts! In the event of highs and lows, the transmitter will vibrate to notify the user, even if the phone is out of range – a particularly useful feature for individuals who are visually impaired or have trouble hearing.
This is the first CGM to provide on-body vibration alerts! In the event of highs and lows, the transmitter will vibrate to notify the user, even if the phone is out of range – a particularly useful feature for individuals who are visually impaired or have trouble hearing. -
Eversense has very strong accuracy, especially since it minimizes the number of day 1’s – the early period right after a sensor is inserted when it is the least accurate. A “day 1” only occurs once every 90 days with Eversense, instead of every 7-14 days with other CGMs.
-
Acetaminophen (Tylenol) does not interfere with the sensor.
The system is compatible with Android and Apple devices. Glucose readings are sent to the smartphone app every five minutes from the on-body transmitter. There is no separate receiver for Eversense – the smartphone app is the only way to view data.
Pricing for the system is expected to be similar to other CGMs in the US. According to Senseonics, there will be “some insurance coverage” within 2018 and “the majority” in the next two years.
This expected approval comes after a unanimously positive March FDA advisory panel, where health professionals, patient advocates, and study participants strongly argued for approval (diaTribe included). We are excited to see FDA approve a new CGM option in the United States and look forward to an upcoming study this summer for the 180-day Eversense XL version, which has already been approved in Europe.







