Tech Watch: The Latest in Diabetes Tech News

With so many things happening in the diabetes tech world, it can be hard to keep up. Stay up to date here with the latest diabetes tech news and alerts.
Color-Changing, Needle-Free Glucose Sensor Gets FDA Nod

Many people with type 2 diabetes find monitoring blood sugar at home a pain. Even though continuous glucose monitoring (CGM) reduces the frequency of fingersticks, it still requires inserting a sensor under the skin, which can be uncomfortable for some people.
The FDA has cleared a new glucose sensor called the Biolinq Shine, a needle-free option for people with type 2 diabetes who don't use insulin. The Shine is an adhesive patch about the size of a quarter that uses a small, color-coded LED indicator, rather than a numeric display, to show whether glucose is in, above, or below range.
Cleared for adults ages 22 and up, it also tracks other measures that may support glucose management, like sleep and activity, with details viewable in a mobile app.
While not as precise as the numerical data a CGM offers, new approaches like the Biolinq Shine offer people with type 2 diabetes not on insulin an easy, needle-free way to monitor their blood sugar.
Published October 6, 2025
New Medtronic CGMs Now Available for AID Use
Until now, users of the MiniMed 780G automated insulin delivery (AID) system had just one integrated CGM option: the Guardian 4.
Now, two new Medtronic sensors – the 6-day Simplera Sync and the 15-day Instinct (built on Abbott’s FreeStyle Libre technology) – are available in the U.S. Both integrate with the 780G for automated insulin delivery in people with type 1 diabetes.
Medtronic users now have greater flexibility when choosing a CGM. The 780G was recently approved by the FDA for use in adults with type 2 diabetes, paired with Guardian 4 or Simplera Sync. Instinct is approved for type 1 diabetes but not yet approved for type 2 use.
Published September 16, 2025
Roche Receives Approval in Europe for CGM and mySugr App

Using continuous glucose monitoring (CGM) often leads to greatly improved diabetes management, but readings are only part of the equation. Glucose predictions can help people get ahead of highs and lows – before they occur.
Roche recently received approval in Europe to pair its Accu-Chek SmartGuide CGM with the mySugr app. The move brings SmartGuide’s forecasting into an app used by many people with diabetes worldwide. The algorithm provides 30-minute and two-hour forecasts, along with a seven-hour overnight low-risk prediction.
Recent study data showed the system’s Night Low Predict feature was associated with a 20% reduction in any nighttime hypoglycemia and a 31% reduction in severe (less than 54 mg/dL) hypoglycemia at night.
Based on new research, pairing the SmartGuide CGM with the mySugr app could help reduce hypoglycemia risk and reduce diabetes distress in an app familiar to many people with diabetes. Currently only available in select countries, Roche is planning a bigger rollout of its CGM to more than 30 countries by the end of 2025.
Published September 9, 2025
AI Plus Coaching May Dramatically Improve A1C in Type 2

Type 2 diabetes affects 1 in 10 Americans, and many struggle to reach their glucose goals.
A new Cleveland Clinic trial tested a program called Twin Health, which combines AI insights with support from a health coach. After a year, 71% of participants with type 2 lowered A1C below 6.5% while using only metformin, compared to 2.4% receiving their usual care. They also lost more weight – an average of 27 pounds – and cut back on other diabetes drugs.
The program built a personalized virtual model of each participant – called a "digital twin" – using daily data from wearable devices. That model predicted how each person would respond to food, activity, and sleep, and then delivered recommendations through an app and with the help of remote coaches.
Twin Health is free to participants but only offered through employers or insurers. The program covers a scale, CGM sensors, a blood pressure monitor, and a Garmin smartwatch at no out-of-pocket cost.
Personalized guidance on food, activity, and sleep helped many people in the study achieve outcomes that look a lot like remission – and reduced reliance on medications.
MiniMed 780G Now Available for Type 2 Diabetes
Published September 2, 2025
 Until this year, automated insulin delivery (AID) systems weren't available for people with type 2 diabetes who take insulin.
Until this year, automated insulin delivery (AID) systems weren't available for people with type 2 diabetes who take insulin.
Medtronic’s MiniMed 780G AID system has just been given the green light by the FDA – the system now joins Tandem (t:slim X2 and Mobi) and Insulet (Omnipod 5) in offering AID technology for adults with type 2.
In addition, the FDA has also cleared Medtronic's upcoming Instinct sensor (made by Abbott) to integrate with the 780G. Instinct, a CGM designed for type 1 diabetes, is expected to be available sometime this year.
These approvals give people with type 2 another option for diabetes tech to improve glucose management. And once Medtronic’s new Instinct and Simplera sensors become available later this year, current 780G users will soon have three different CGM choices.
Omnipod 5 to Sync With FreeStyle Libre 3 Plus CGM
Published August 18, 2025
Insulet recently announced that its Omnipod 5 automated insulin delivery (AID) system will pair with Abbott's FreeStyle Libre 3 Plus continuous glucose monitor (CGM) sometime in 2026.
The hybrid closed loop system currently supports the Libre 2 Plus CGM. The newer Libre 3 Plus is Abbott's most advanced system. It offers glucose readings every minute, a 14-day wear time, and is FDA-approved for use in people with diabetes ages 2 and above and in pregnancy.
Omnipod users who prefer the Libre CGM are likely to welcome the smaller footprint of the latest sensor, which is about the size of two stacked pennies. By comparison, the previous generation Libre 2 Plus is about the size of two stacked quarters.
Insulet has not yet announced when the Omnipod 5 app will support Libre CGMs. Current Omnipod 5 users who prefer the Libre 2 Plus use the AID system's controller. Insulet has been expanding choices for users recently, including adding support for the Dexcom G7 in the Omnipod 5 Mobile app in June 2025.
Medtronic Shares Sneak Peek of New Abbott CGM Sensor
Medtronic is adding a new CGM option for their automated insulin delivery (AID) system and smart insuliin pen. The company recently provided a first look at “Instinct,” a new sensor developed by Abbott to be used with the MiniMed 780G or the InPen.
More details are to come on a commercial launch date, but Medtronic is planning on submitting Instinct to the FDA for clearance sometime in 2025.
Tandem Issues Medical Device Correction for t:slim X2
Published August 11, 2025
Tandem recently issued a medical device correction for t:slim X2 insulin pumps with a speaker-related malfunction. Speaker failures may cause errors that can stop insulin delivery and terminate communication between the pump and CGM, which could lead to hyperglycemia.
 Tandem has sent a letter to all customers with impacted devices. If you have received a letter or your pump gets a Malfunction 16 error, the pump is no longer operational, and you should switch to a backup insulin delivery method until the pump can be replaced. To find out if your device is impacted, check the serial number and see if it’s listed on Tandem’s site here.
Tandem has sent a letter to all customers with impacted devices. If you have received a letter or your pump gets a Malfunction 16 error, the pump is no longer operational, and you should switch to a backup insulin delivery method until the pump can be replaced. To find out if your device is impacted, check the serial number and see if it’s listed on Tandem’s site here.
If you receive a Malfunction 16 alert, contact Tandem at 1-877-801-6901 or techsupport@tandemdiabetes.com for a replacement. Even if you haven’t had a Malfunction 16, it can occur at any time. Tandem advises using the t:slim mobile app with push notifications turned on, so if the malfunction does occur, you’ll be notified.
Tandem said it will be releasing a software update that enhances early detection of speaker failure. The update will also include persistent vibration alerts to help reduce potential safety risks.
Tidepool Now Syncs With Abbott FreeStyle Libre CGMs
Published August 3, 2025
 Tidepool, a nonprofit website, recently announced that its diabetes data visualization service will sync up with Abbott FreeStyle Libre 2 and 3 continuous glucose monitoring (CGM) systems.
Tidepool, a nonprofit website, recently announced that its diabetes data visualization service will sync up with Abbott FreeStyle Libre 2 and 3 continuous glucose monitoring (CGM) systems.
The upgrade will make it easier for users of Libre CGM to see their blood sugar, treatment, food, and activity data all in one place – without cables or manual uploads. Until recently, Libre users had to use a desktop app and a USB connection to upload their info.
This new update skips all that. Once users link their free Tidepool Web and LibreView accounts, they can see time in range, carbs, activity, and insulin doses all in one view – and share it with their health providers.
Bringing all this data together for Libre users – without cables or uploads – makes spotting trends and sharing health information with their care team much easier.
Missed Connections: Open-Source AID Users Have Trouble Pairing With Pods
Published July 30, 2025
Users of open-source automated insulin delivery (AID) software like Loop have reported problems after upgrading to the iPhone 16 and connecting to Omnipod DASH pods.
Several open-source hybrid closed loop systems pair with Insulet's previous generation pods, but the developers of Loop and other systems acknowledge there are Bluetooth connection hiccups when attempting to connect with the latest version of the DASH pods.
Luckily, the pods do pair with Apple's latest iPhone, but users say it can take some time. For example, iPhone 16 users may have to make numerous attempts to initiate (aka pair) with their new pod after filling it with insulin. When users try to deliver a mealtime insulin dose or blood sugar correction (called a bolus), the command can fail repeatedly before working. Users have come up with workarounds like cycling Bluetooth on and off on their iPhone and restarting the phone. It's unclear if these actually help or if it just takes time for the phone and insulin pump to connect.
Developers of open-source AID systems that use the pods have posted some tips for those with iPhone 16 connection issues. However, the apps – including Loop, Trio, and iAPS – aren't FDA-approved or supported by Insulet, and Omnipod 5 pods aren't compatible with open-source systems. Users who use open-source apps will unfortunately need to be patient as the developers who keep the programs up to date come up with a fix.
Omnipod 5 iPhone App Now Works With Dexcom G7
Published July 21, 2025
 Those who use the Omnipod 5 and Dexcom G7 CGM can now manage the automated insulin delivery system with their iPhones. This new app update means people with diabetes have one less device to keep track of and carry.
Those who use the Omnipod 5 and Dexcom G7 CGM can now manage the automated insulin delivery system with their iPhones. This new app update means people with diabetes have one less device to keep track of and carry.
Until recently, Omnipod 5 users needed a separate controller to manually deliver insulin, adjust settings, change the tubeless pump – called a pod – and view glucose readings and trends. Now with G7 support, Omnipod 5 users can manage their diabetes all with their iPhone. Once a pod is attached to the body, the algorithm runs from the pump itself, adjusting insulin doses based on glucose levels.
Insulet had previously added support for Android and Dexcom G6 users who wanted to use their phones to run the system. The updated app is available here in the Apple App Store.
New Twiist AID System Now Available

Published July 14, 2025
After over a year long wait, the twiist is now officially available in select areas in the U.S. The automated insulin delivery system uses Tidepool's Loop algorithm, which is embedded in a circular, tubed pump. The system's pump uses a novel way to measure insulin with sound waves – an approach that may allow for more accurate dosing. It’s also the first FDA-approved AID system that can be directly controlled by an Apple Watch.
Roughly 4,000 people on a waiting list will be prioritized during a regional rollout, with nationwide availability expected by year’s end. The price is capped at $50 per month with insurance. The first month of supplies – including the pump and infusion sets – are free. Users will need a compatible CGM, such as the Abbott Freestyle Libre 3 Plus and Eversense 365.
The twiist system’s unique ability to accurately measure insulin could better help keep blood sugar levels in range. The system also adds a new choice for people with diabetes, including those currently using an open-source version of Loop, who will now have an FDA-approved, commercially available option.
 Hands-Off AID System Improves Teens’ Glucose Levels
Hands-Off AID System Improves Teens’ Glucose Levels
Published June 9, 2025
Teens with type 1 in the UK showed significant improvement in a new study, after switching from standard insulin pump and continuous glucose monitoring to a fully closed loop insulin delivery system (AID).
The participants, ages 13 to 19, began the study with above-target A1C levels – averaging 8.9%, compared to the recommended 7.5% for teens. Before starting the automated system, they spent nearly 40% every day with blood sugar levels above 250 mg/dL.
But when they switched to using a fully closed loop system, their time in range (70-180 mg/dL) increased by 13% – without announcing meals or manually delivering boluses. Time in tight range (70-140 mg/dL) also increased, from 19% to 29%.
Participants used the CamAPS HX AID system with Fiasp, an ultra-rapid insulin. Along with the improved time in range, there was no increase in time below range, no severe low blood sugar events or episodes of diabetic ketoacidosis reported. The fully closed loop system also reduced diabetes distress.
Researchers concluded that fully closed loop systems may be a safe and effective option for teens who struggle with managing their blood sugar.

Twiist AID Will Pair With Abbott’s Dual Glucose-Ketone Sensor
Published May 20, 2025
Sequel, maker of the new twiist automated insulin delivery system, recently announced the device will pair with a future glucose sensor from Abbott that also monitors ketones, greatly reducing the risk of diabetic ketoacidosis. DKA is a serious complication of diabetes that can happen when insulin levels drop too low.
The twiist, available later this year, will offer the first FDA-approved Tidepool Loop-based algorithm. Loop is popular with open-source users but requires a relatively complicated setup. The algorithm for the twiist AID system lives on the pump itself, greatly simplifying starting up.
The twiist's insulin pump can precisely measure the volume and flow of insulin, reducing the possibility of injecting air, which can lead to unexpectedly high blood sugar levels. The pump can also deliver micro doses of insulin, as small as 0.3 units, to those who are very sensitive to insulin, including children ages six and older.
Earlier this year, Sequel announced twiist will pair with Abbott's Freestyle Libre 3 Plus CGM and the Eversense 365, an implantable CGM sensor that lasts a full year.
 FDA Clears Dexcom's G7 15 Day for Adults
FDA Clears Dexcom's G7 15 Day for Adults
Published April 15, 2025
Dexcom's next-generation G7 15 Day continuous glucose monitor has been approved by the FDA for people with diabetes ages 18 and older. The company said the CGM system should be available in the second half of 2025.
In a study presented at the ATTD 2025 conference in March, the G7 15 Day was shown to be slightly more accurate than the current G7. The device looks the same, uses the same receiver and should have the same monthly cost and copay as the company's 10-day G7 CGM. However, a new prescription will be required to purchase it.
By launch, the longer-lasting CGM should integrate with the AID systems that use the current G7, Dexcom said, such as the iLet Bionic Pancreas, Omnipod 5, and Tandem t:slim X2 and Mobi. Work is still being done to ensure the Dexcom G7 15 can be used by children, Dexcom added. The current G7 CGM is FDA cleared for those 2 years and older.
Virtual Programs Help Lower Blood Sugar
Published April 7, 2025
People with type 2 diabetes in a recent study saw their A1C drop significantly (on average from 8.9% to 7.9%) using different online tools to help manage their care. And most participants in the trial, conducted at the Texas A&M University School of Public Health, stuck with the program for its entire 6-month run.
One strategy involved a virtual, self-paced class that included one-on-one coaching sessions with a nurse or dietitian. Another used a smartphone app with self-guided education and a chat feature to connect with a diabetes coach. The third combined both strategies. All three were found to be similarly effective. The study included people with type 2 from rural and urban areas in Texas.
The results suggest that varied online education and personal coaching can help significantly lower average blood sugar levels, reducing the risk for short and long-term health issues related to diabetes.

AID Use in Kids Can Help Reduce Family Stress
Published April 1, 2025
Dr. Carine de Beaufort, a pediatric endocrinologist at the Centre Hospitalier du Luxembourg, at ATTD 2025 in Amsterdam presented data from multiple studies showing how automated insulin delivery systems (AID) improve glycemic control while reducing stress and worry related to diabetes management that affects an entire family.
"Let life be less about diabetes and more about life," de Beaufort said, pointing to research on how AID systems alleviate diabetes burden by automating many of the time consuming aspects of carb counting and blood sugar control.
Young children using the CamAPS system in Europe over 18 months, she said, consistently reported better sleep, and parents spent less time worrying about their child's blood sugar while they slept. In another study, the Omnipod 5 AID system was shown to be safe and effective at improving blood sugar control and reducing hypoglycemia in children as young as 2 years old.
These results are part of the reason de Beaufort supports the use of AID systems in children younger than 6 from the time of diagnosis, she said.
"Parents are more at ease, knowing it works well," she said. "And children are able to play and sleep without being interrupted."
Dexcom Receives Warning From FDA for Manufacturing Issues
Published March 17, 2025
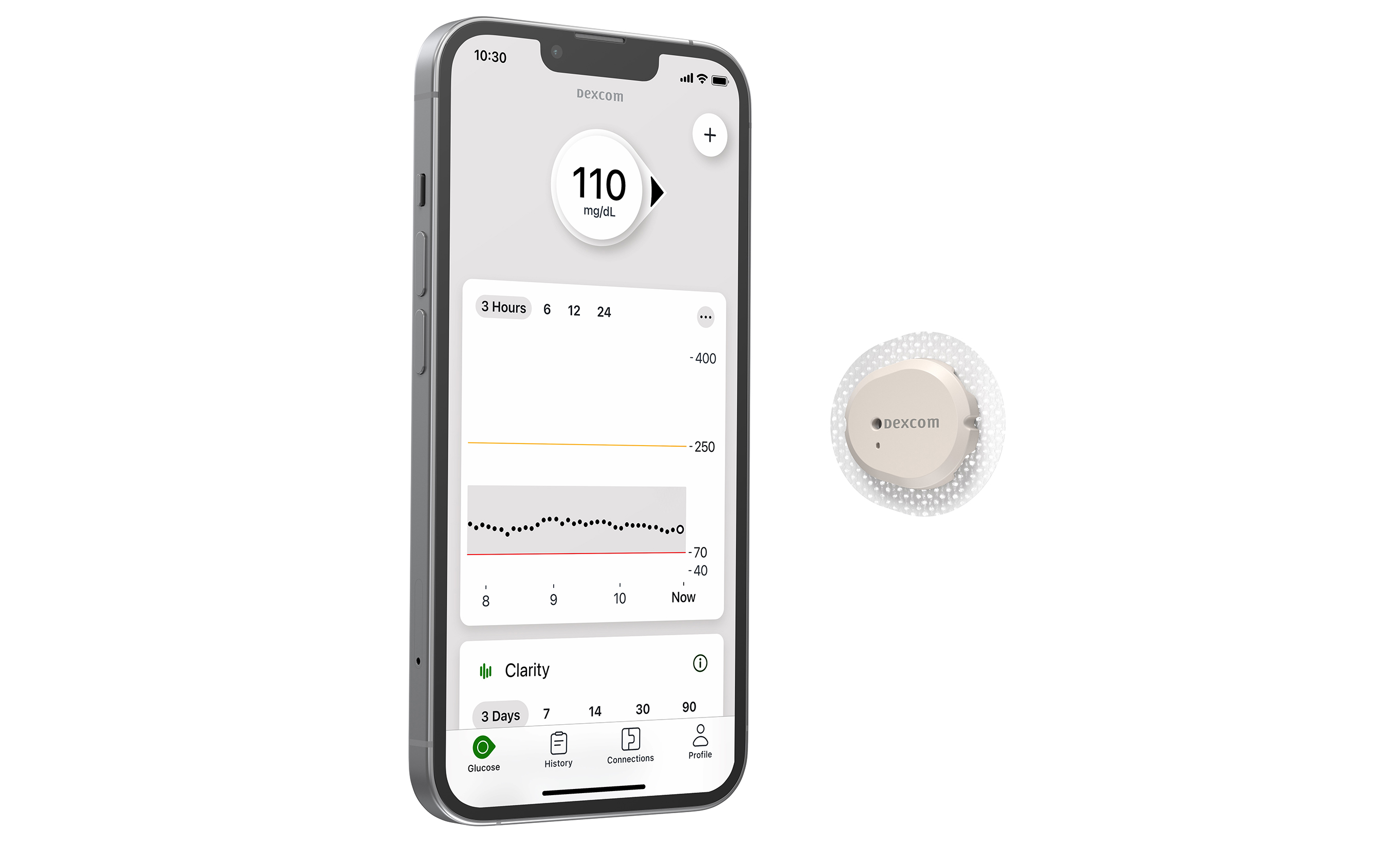 Dexcom recently received a warning letter from the FDA after inspections of their CGM manufacturing facilities in San Diego, California, and Mesa, Arizona in 2024. The FDA issued the letter due to “non-conformities in manufacturing processes and quality management systems” and cited deficiencies in Dexcom’s response letters to the FDA.
Dexcom recently received a warning letter from the FDA after inspections of their CGM manufacturing facilities in San Diego, California, and Mesa, Arizona in 2024. The FDA issued the letter due to “non-conformities in manufacturing processes and quality management systems” and cited deficiencies in Dexcom’s response letters to the FDA.
The full details behind the manufacturing issues aren’t yet known, but Dexcom has not been asked to recall any products or halt manufacturing or distribution of their CGM products. The warning letter also hasn’t impacted applying for clearance for any new products, including the 15-day Dexcom G7, which is currently under review by the FDA. Dexcom has acknowledged the seriousness of the warning and is working on preparing a written response; until the issues cited by the FDA are resolved, Dexcom could face additional regulatory or legal action.
Though Dexcom CGM products aren’t said to be affected, it’s always a good idea to double (maybe even triple) check your devices are working as they should. See our resource guide here for ensuring you receive critical blood sugar alerts, as well as common CGM hang-ups and how to get around them.
Medtronic Issues Medical Device Correction for InPen
Published March 10, 2025

Medtronic has issued a medical device correction for certain InPen smart insulin pens due to an assembly issue. Some InPens from specific lots may have defects that cause difficulty in either inserting the insulin cartridge into the holder or removing the cartridge holder from the pen.
If excessive force is required to remove the cartridge holder, or if the insulin cartridge is not fitting into the cartridge holder, Medtronic advises that the InPen should not be used.
To find out if your InPen is impacted, check the lot number and see if it’s listed on Medtronic’s site here. If your InPen is affected, call 1-800-646-4633 (option 1) or visit this website to request a replacement. Affected InPens should not be used.
Tandem's AID Tech Cleared for Type 2 Diabetes
Published March 3, 2025
This spring, people with type 2 diabetes will have more choices when starting with an automated insulin delivery system. The FDA recently cleared Tandem's next-generation Control-IQ+ AID technology for people with type 2 who are 18 years and older.
The AID systems, which use the t:slim X2 and Tandem Mobi insulin pumps, had previously only been available to people with type 1 diabetes for ages 6 years and older.
The new Control-IQ+ algorithm should also be available for current users of Tandem AID systems in March. Improvements in the latest version of the algorithm are expected to include new capabilities for corrective boluses, setting temporary basal rates, and extended boluses.
The company plans to release results from a study of 319 type 2 participants who used the t:slim X2 pump for automated insulin delivery, at the upcoming International Conference on Advanced Technologies & Treatments for Diabetes in Amsterdam, in March.
The Tandem AID systems join Insulet's Omnipod 5, which is also approved for use in type 2 for people who are 18 years or older.

Free Exercise App Sweats the Details
Published February 25, 2025
People with diabetes know exercise can be a challenge. Certain aerobic activities, like walking, biking, or swimming, can be a great way to lower blood sugar. However, the effects of exercise can be unpredictable, sometimes requiring fast-acting sugar to correct hypoglycemia. On the flip side, other forms of physical activity like anaerobic exercise (weight lifting, sprinting, and interval training) can initially cause blood sugar to rise. These blood sugar variations – and chasing workouts with calories – can lead to frustration.
A free app called Enhance-d aims to make exercise while managing type 1 and type 2 diabetes easier. Available for iPhone and Android, the app pulls health-related diabetes data into one place through a series of colorful graphs.
The app also integrates data from wearable devices that track fitness, like those from Fitbit and Garmin, or the Oura ring, which can record heart rate, sleep quality, and stress data. The Enhance-d app pulls data from these various health devices and apps and produces reports showing how different physical activity, meals, insulin doses, sleep patterns, and other factors affect time in range.
These reports and related analyses provided by the app can also be shared with a healthcare provider to identify trends. That data could potentially help inform modifications to exercise intensity or other adjustments to improve blood sugar management and overall health.
 Watch for Insulin Pump Over-and-Under Dosing When Flying
Watch for Insulin Pump Over-and-Under Dosing When Flying
Published February 18, 2025
As diaTribe reported in September, changes in cabin pressure during air travel can cause insulin pumps to over and under-deliver about half a unit of insulin during takeoffs and landings.
After receiving reports about the issue and confirming during tests, Medtronic recently issued a safety alert for users of the company's insulin pumps to monitor their glucose when flying. The following pumps were listed in the alert: Paradigm, MiniMed 630G, MiniMed 670G, MiniMed 770G, and MiniMed 780G.
Researchers at the 2024 EASD conference showed insulin pumps over-delivered 0.6 units of insulin during tests that simulated ascent. During descents, the pumps under-delivered 0.5 units. The researchers tested pumps from Medtronic, Insulet, and Tandem.
They found that the drop in cabin pressure as the plane ascends can cause bubbles in the cartridge, which may lead to a small amount of insulin being delivered unintentionally. Then on descent, a small reduction in insulin delivery can occur, which could cause an increase in blood sugar.
To avoid these issues, the researchers recommended disconnecting the pump just before takeoff and reconnecting 20 minutes later. Once cruising altitude is reached, the researchers suggested users remove any bubbles from the cartridge as they would during filling or manually priming the pump. For pumps that can't be disconnected, like the Omnipod, eating a very small snack if needed during takeoff could help. On landing, eat another small snack followed by a bolus to avoid a rise in blood sugar.

FDA Issues Warning About Missed Diabetes Device Alerts
Published February 10, 2025
The FDA has issued a warning that users of continuous glucose monitors have reported missing critical smartphone alerts. The agency is recommending CGM users check their systems to make sure they can hear the alarms, especially after settings changes and operating system upgrades.
Users have reported missed urgent alerts of low and high blood sugar when they thought their phones were set correctly to deliver alarms.
“Even if configured correctly, certain hardware or software changes can interrupt the expected operation of these critical devices, which can lead to patient harm if undetected,” said Courtney Lias, director of the Office of In Vitro Diagnostic Products in the FDA’s Center for Devices and Radiological Health, in the announcement.
The FDA reported missed alerts may have contributed to serious harm, in some cases fatal, including hypoglycemia, severe hyperglycemia, and diabetic ketoacidosis.
Reports to the FDA involved multiple devices, with users reporting missed alerts at night, while driving, and while wearing headphones. In some cases, users reported problems with CGM apps installed on operating systems and smartphone devices that weren't supported by the manufacturer. In other cases, the devices were set in do-not-disturb or focus mode, or were in sleep mode for an extended period of time.
The FDA is recommending users take steps to make sure their CGM alerts are working as expected:
- Turn off automatic operating system upgrades, and confirm with the device manufacturer that any operating system upgrades are compatible with the current version of the device apps before installing.
- You may need to wait to install operating system upgrades until the device manufacturer releases a compatible version of the app.
- Note that connecting to accessories, such as wireless headphones or car audio systems, may lower the volume of alerts, or the alerts may not work at all.
- Check smartphone alerts at least once a month to confirm they're working as intended.
- Follow the instructions from the device manufacturer on installing, setting up, and updating medical apps.
- If alerts are not being delivered as expected, contact technical support for the device.
- Report any unresolved problems with diabetes devices to the FDA.
The FDA said it is working with device manufacturers to ensure that safety alerts are carefully tested before being used by people with diabetes. The agency also said it will continue working with device makers to ensure alerts are continuously evaluated, with necessary updates quickly communicated to users.
ADA Releases New Guide for Automated Insulin Delivery
Published February 3, 2025

The American Diabetes Association recently published new guidelines for automated insulin delivery (AID). While the guide is meant for healthcare providers, it provides practical tips and considerations that would benefit anyone considering adopting the technology.
For example, the guide recommends setting aside plenty of time for the learning curve associated with starting a new AID system (so no imminent vacations, for example) and recognizing that blood sugar goals won't be reached overnight. Also covered is how to choose a system, including weighing options like the size of the pump or if it uses tubing. The guide also recommends considering whether the user would prefer a simpler device, with less control of some settings, or one that might take more time to learn (and requires more input, like carb counting) but is likely to achieve better time in range.
The ADA document also offers AID system pros and cons. For example, smartphone control (available with some devices) is listed as a pro, since it allows discrete insulin dosing and adjustments. Cons include having the tech attached to your body and equipment failures (the guide recommends keeping nearby backups of insulin and infusion sets or pens/vials.) Read all the recommendations, including considerations regarding mental health.
Photo: Tandem Diabetes
 Beta Bionics Plans Tubeless Patch Pump
Beta Bionics Plans Tubeless Patch Pump
Published January 27, 2025
Diabetes technology maker Beta Bionics is developing a tubeless patch pump they plan to launch in 2027 as part of its automated delivery system, the iLet Bionic Pancreas.
The company reported the device has two parts. One reusable component holds the electronics that operate the device and motor. A second disposable part includes an adhesive patch, insulin reservoir, insertion device, and the cannula used to deliver insulin.
Patch pumps use an adhesive to adhere to the skin directly. Currently, the Omnipod is the only tubeless patch pump for use in people with type 1 and type 2 diabetes. Small pumps like the Tandem Mobi and upcoming twiist pump use a thin tube and cannula to deliver insulin. These small devices can be inserted into a sleeve with adhesive on the back that sticks to the skin, resembling a patch pump.
Beta Bionics said the pump is planned for use in people with type 1 diabetes and later will expand its use for people with type 2. The company also reported it is continuing work on an AID system that would, in addition to using insulin to lower blood sugar, also contain glucagon to raise blood sugar. Research is being conducted into dual-hormone systems, but none are available yet for people with diabetes.
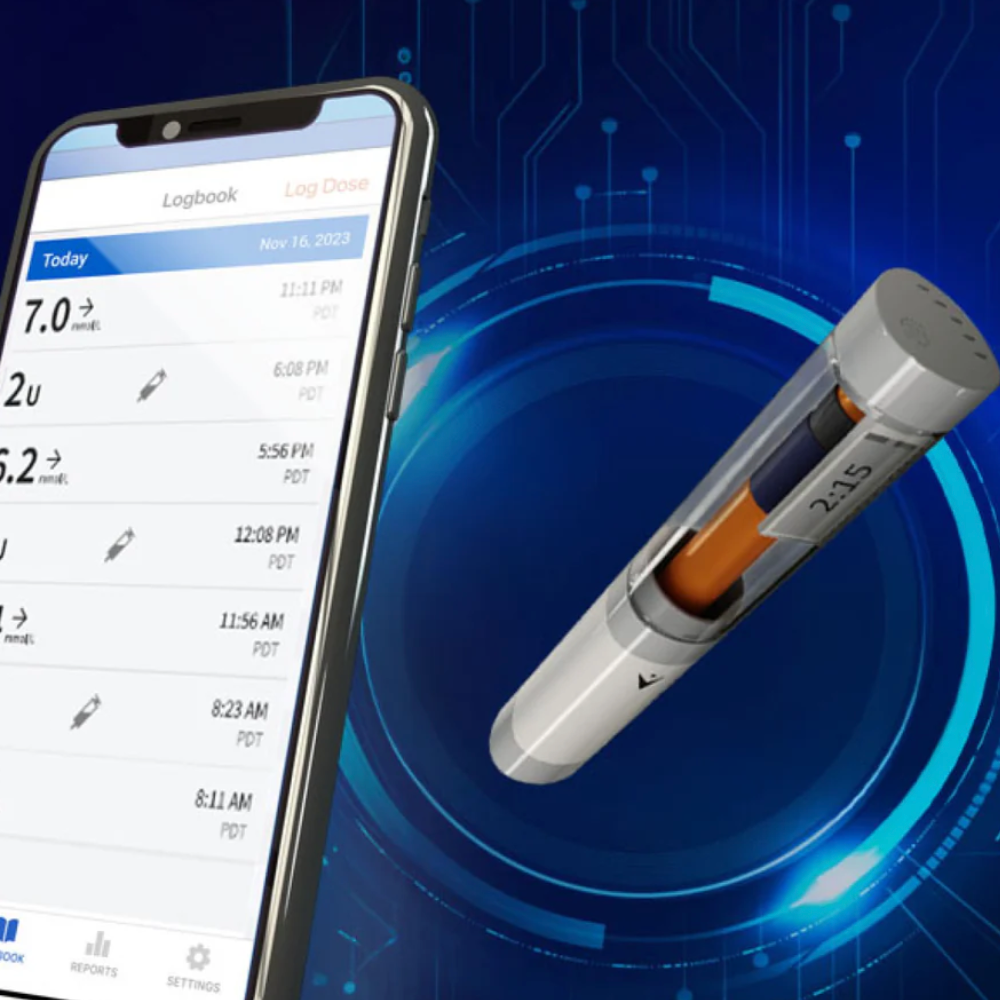
Smart Pen Cap Tracks Doses and Helps Insulin Keep Its Cool
Published January 21, 2025
At the CES technology conference in Las Vegas this month, a company called TempraMed demonstrated its upcoming VIVI Cap Smart device that protects insulin from extreme temperatures and tracks doses via smartphone.
 When exposed to heat, insulin can break down and lose its effectiveness. The FDA says when not stored in a refrigerator, insulin is safe to use for about a month at 59-86 degrees Fahrenheit. Insulin at 98.6 degrees or higher should be tossed out, and insulin that has frozen should not be used.
When exposed to heat, insulin can break down and lose its effectiveness. The FDA says when not stored in a refrigerator, insulin is safe to use for about a month at 59-86 degrees Fahrenheit. Insulin at 98.6 degrees or higher should be tossed out, and insulin that has frozen should not be used.
The VIVI Cap has a layer of thermal insulation and an additional layer of material that absorbs heat – keeping it away from the insulin. This capability reactivates as the temperature cools.
A temperature sensor glows green when pressed if insulin is in a safe range. The company says in its tests the device kept the insulin in a pen safe to use in 100-degree heat for five days.
The VIVI Cap Smart will be available in August. Pricing hasn't yet been announced. The current VIVI Cap (with dose tracking) sells now for $149, however, and the company's website offers a pre-order offer for the new smart device at a discount. The company is also developing a protective device for insulin vials.
Another option for keeping insulin cool are the FRÍO cooling cases ($27 to $39). Read more in our holiday gift guide for people with diabetes.
Dexcom Fixes G7 App for International Travelers
Published January 6, 2025
Dexcom has released an upgrade to its G7 app for iPhones and Androids, which fixes an issue that kept some users from being able to install the app. When traveling outside their home country, users sometimes found they could not install or reinstall the app.
Version 2.6 of the app fixes the problem, allowing users to uninstall and install the G7 app when abroad. "The download is tied to your App Store or Google account's country settings, which don't change when you travel," according to the company. However, the user must have first installed the app for the first time in their country of residence.
Those planning to travel internationally are advised to make sure the app is installed and updated to the latest version before going abroad.
New Virtual Endo Service Now Available
Published January 6, 2025
Health tech company eddii recently launched a service for virtual endocrinology care, which currently offers online visits with pediatric endocrinologists in 40 states and virtual care for adults with diabetes in eight states (see their coverage map).
This year, the company plans to expand the service to serve more children and adults with diabetes.
Users of the app can book appointments, see licensed endocrinologists, and get prescription refills. Users will typically get an appointment for online care within two days, the company said. The quick turnaround could be helpful for people with acute needs like sick-day guidance or who need a prescription refill.
A rapid self-assessment feature helps people decide if they need an appointment. "It tells them whether they need to seek immediate help, should book an appointment with a provider, or just have to wait a few hours and keep an eye on their symptoms," said James Northcutt, chief marketing officer at eddii.
The app's other features aim to make diabetes management more engaging. The service takes CGM readings from a Dexcom continuous glucose monitor and incorporates them into multiple games to encourage improvements in time in range. The app also uses quizzes to engage users around diabetes trivia and education.
A chatbot feature focused on mental health around diabetes offers conversations and health tips. Users can also set up phone calls for alerts when blood sugar is high or low, for up to five people, such as friends, loved ones, or caregivers.
Users can download the app on the Apple App Store or the Google Play store for Android. The eddii app joins the growing list of virtual care platforms to help with diabetes management and improve time in range.
Omnipod 5 Integrates Freestyle Libre 2 Plus CGM
Published November 22, 2024
The Omnipod 5 automated insulin delivery system now works with Abbott’s FreeStyle Libre 2 Plus continuous glucose monitor, Insulet announced this week.
Adding compatibility for the 15-day CGM may appeal to people with type 1 or type 2 diabetes who use Omnipod insulin pumps and prefer Freestyle Libre CGM sensors.
Insulet's Omnipod 5 joins the Tandem t:slim X2 (Freestyle Libre 2 and Dexcom G6/G7) and Beta Bionics’ iLet (Dexcom G6/G7 and Freestyle Libre 3) in supporting both Abbott and Dexcom CGMs.
The Omnipod 5 is a tubeless, waterproof patch pump that can be worn for three days. The pod has an integrated algorithm that can adjust insulin doses based on blood sugar levels every five minutes. The system costs about $50 per month for users with insurance or about $600 without.
Users in the U.K. and Netherlands have had access to the Omnipod 5 and Libre 2 Plus integration since June. More than six million people now use Freestyle Libre sensors worldwide.
Medtronic's Smart Pen App Gets the Go-Ahead in the US
Published November 22, 2024
The FDA recently cleared the app for Medtronic's InPen, a smart device used for multiple daily injections (MDI). The clearance will allow Medtronic to begin a limited release of its smart MDI system, with broader commercial availability to be announced later.
The InPen system allows users to calculate and track their insulin doses, which will help avoid missed doses. The pen connects via Bluetooth to the company's Simplera CGM, which records blood sugar levels every five minutes.
The InPen app can take a blood sugar reading from the Simplera CGM or blood glucose meter and calculate a premeal dose or correction, if necessary, based on the glucose reading, active insulin, and carbs entered before a meal.
The reusable pen lasts for one year without a need to charge. The InPen also monitors insulin temperature to maintain its effectiveness. Medtronic says most people with insurance will pay about $35 for an InPen per year. The full price without insurance is $549.
Medtronic has aimed the smart insulin pen at those who use multiple daily injections because of cost or personal choice. The InPen may also provide a path for those who want to transition from MDI to an AID system.
Smart Ring Oura and Dexcom Pair for Health Tracking
Published November 22, 2024
Dexcom and smart ring maker Oura have announced plans to integrate continuous glucose monitoring with health and fitness data. The readings will appear in the Oura and Dexcom apps to shed light on how sleep, stress, vital signs, and other factors affect blood sugar levels.
 The Oura ring is worn on your finger and tracks a wide range of health and fitness data, including motion, heart rate, and heart rate variability, which the company uses to track sleep quality and measures. The ring also measures body temperature and motion, which combined with heart data, helps monitor stress levels. The company says temperature can also be used to track menstrual cycles, which can impact glucose levels.
The Oura ring is worn on your finger and tracks a wide range of health and fitness data, including motion, heart rate, and heart rate variability, which the company uses to track sleep quality and measures. The ring also measures body temperature and motion, which combined with heart data, helps monitor stress levels. The company says temperature can also be used to track menstrual cycles, which can impact glucose levels.
The initial app with integrated data from the ring and CGM is planned for the first half of 2025. The Oura ring sells for between $299 and $449 (depending on the style).
Omnipod 5 App for iPhone Now Available
Published November 21, 2024
The iPhone app for the Omnipod 5 is now available for download in the Apple App Store. The new software is an upgrade for Insulet Omnipod 5 users who will now be able to carry one less device, called the controller, to operate the automated insulin delivery system.
"With the iPhone app, a user can deliver a bolus of insulin for a meal, change a pod, and check CGM values and trends," said Eric Benjamin, Insulet’s executive vice president and chief product and customer experience officer.
Also new is a "custom foods" feature that allows users to save frequent meals to reduce manual carb counting. To use the app, Omnipod 5 users will need iOS 17 or 18 and a Dexcom G6 continuous glucose monitor.
The iPhone version joins Insulet's Android app that was released last May.
Tidepool Will Support Abbott's Freestyle Libre CGM Devices
Published October 28, 2024
Software company Tidepool announced Monday that it will integrate data from Abbott's FreeStyle Libre continuous glucose monitors (CGM) into Tidepool+, an online tool for viewing data on a wide range of diabetes devices.
Tidepool+ provides a way for users of diabetes tech to see insulin pump, CGM, and glucometer data in one place. That data can then be easily shared with healthcare providers.
Blood sugar readings and treatment information can be uploaded to Tidepool+ from supported devices and the Tidepool Mobile app. Users can also manually upload data from other devices. Now Abbott’s FreeStyle Libre has been added to the list, allowing users to seamlessly integrate their Libre data into the Tidepool+ online tool.
No timing has been announced yet for when FreeStyle Libre CGM data will be able to be integrated into Tidepool+.
New Bionic Pancreas App Released
Published October 23, 2024
Beta Bionics, manufacturer of the iLet Bionic Pancreas automated insulin delivery (AID) system, recently introduced the Bionic Circle app, which allows up to 10 people to remotely follow a user's blood sugar, meal, and dosing data. An iPhone version was released last month and the app is now available for Android smartphone users.
The Bionic Circle App sends data from the iLet AID system to the cloud, which can then be viewed by those who accept an invitation to use the app on their phone. The app then remotely displays CGM readings, meal announcements, insulin doses, and alerts for high and low blood sugar.
The app's release for Android will be especially helpful for parents, caregivers, and loved ones who want to see iLet pump and CGM data regardless of whether they're using an iPhone or Android device. The free app can be downloaded from the Apple App store or Google Play.
Medtronic Recalls Insulin Pumps Due to Battery Issue
Published October 23, 2024
Medtronic has issued a voluntary recall of MiniMed 600 or 700 series insulin pumps due to a battery issue that may occur when devices are dropped or receive a jarring impact.
Pumps dropped just once could provide a shorter-than-expected battery life, even after the battery is replaced. A damaged pump could fail to alert the user with the typical amount of battery life remaining, which could stop insulin delivery prematurely. If insulin delivery is stopped unexpectedly, hyperglycemia or diabetic ketoacidosis could result.
Users are advised to replace the battery as soon as the "low battery pump" alarm sounds. Medtronic also recommends that MiniMed pump users carry extra AA batteries with a full charge. If you’re experiencing significantly low battery life, contact Medtronic to see if a new pump is needed. The company said it would replace any pump experiencing the issue at no charge.
Customers in the U.S. can call the Medtronic support line 24 hours a day at 1-800-378-2292. Customers outside the U.S. should use the Medtronic international contacts page.