Omnipod 5 Cleared by the FDA
By Matthew GarzaHanna GutowKatie Mahoney
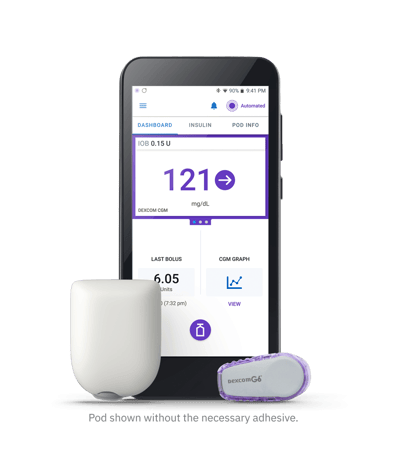 The Omnipod 5 automated insulin delivery system has been cleared by the FDA for people with type 1 diabetes. The system, which includes a tubeless insulin pump, a Dexcom G6 continuous glucose monitor, and a smartphone app, will be available later in 2022.
The Omnipod 5 automated insulin delivery system has been cleared by the FDA for people with type 1 diabetes. The system, which includes a tubeless insulin pump, a Dexcom G6 continuous glucose monitor, and a smartphone app, will be available later in 2022.
The long-awaited FDA clearance of Insulet’s Omnipod 5 automated insulin delivery (AID) system is finally here. The new AID system was initially cleared in January for people with type 1 diabetes ages 6 and up. This month, the clearance was expanded for those ages 2 and up.
“FDA clearance for Omnipod 5 was based on a pivotal trial that found that Omnipod 5 was safe, effective, and demonstrated the trifecta of clinical results including sustained reduction in A1C, improved Time in Range (TIR), and very low occurrence of hypoglycemia,” said Angela Wiczek, senior director of corporate communications at Insulet.
In the Omnipod 5 pivotal trials, children aged 6 to 13 improved their average TIR from 52% to 68% (almost four more hours per day in range) and their A1C from 7.7% to 7.0%. Adults 14 years and older improved their average TIR from 65% to 74% (over two additional hours per day in range) and their A1C from 7.2% to 6.8%. Both groups also showed major decreases in the amount of time spent above range (above 180 mg/dL).
On Aug. 22, the FDA cleared the system for young children ages 2-5. In a recent study of the Omnipod 5 system in this age group, the system helped increase Time in Range by an average of 2.6 hours per day. Also, throughout the entire 13-week trial, there was not a single instance of severe hypoglycemia or diabetic ketoacidosis (DKA) in children using the Omnipod 5 system.
What is the Omnipod 5?
The Omnipod 5 consists of three components:
-
The Pod: A tubeless, wearable insulin pump with the AID algorithm embedded into it. The Pod can be worn for up to three days and can hold 200 U of rapid acting insulin.
-
A Dexcom G6 continuous glucose monitor (CGM).
-
Omnipod 5 mobile app that comes pre-downloaded on a controller provided by Insulet or can be downloaded onto a compatible Android smartphone. The Omnipod 5 app allows the user to have full smartphone control over both basal and bolus rates for the system.
All AID systems require an algorithm that allows the CGM and the pump to talk to one another to adjust insulin dosing based on a person’s glucose levels. In Omnipod 5, the algorithm – called SmartAdjust – is embedded into the Pod itself.
This is the first AID system with the algorithm integrated into the pump, allowing the user continue in automated mode, even if the smartphone or the controller is not within range or loses battery. This is also the first AID system with a patch pump versus a pump with tubes and the first AID system to give the user full smartphone control.

The “SmartAdjust technology… automatically increases, decreases, or pauses insulin delivery, every five minutes, using a customizable glucose target, helping to protect against highs and lows, keeping glucose in range, even while sleeping,” said Wiczek.
The user can customize their glucose target in 10 mg/dL intervals from 110 to 150 mg/dL and the system's algorithm takes into account the amount of insulin on board (the insulin in the body) when making these adjustments.
The Omnipod 5's operating modes
The system can be operated in several modes:
-
Automated mode: Adjusts insulin delivery automatically and requires a CGM connection.
-
Manual mode: Delivers insulin at pre-programmed rates regardless of whether the CGM is connected.
-
Activity mode: Allows the user to temporarily set the glucose target for 150 mg/dL and suspend insulin delivery. This mode is designed for exercising or other periods of increased insulin sensitivity or when there is a higher risk for hypoglycemia.
The CGM and Pod are connected via bluetooth and since the SmartAdjust algorithm is housed within the Pod, even if the controller or your phone were to run out of power (or move outside of the bluetooth range), the system can still run in automated mode.
When will the Omnipod 5 be available?
The Omnipod 5 system is now available to everyone with a prescription through their pharmacy.
How much will the Omnipod 5 cost?
The Omnipod 5 app (on the provided controller or as a smartphone app) will be provided at no additional cost. The Pod prescription price will vary depending on individual insurance coverage.
Insulet indicated that the Omnipod 5 is priced the same as the Omnipod DASH and already has significant insurance coverage (comparable to that of the Omnipod DASH), Insulet’s currently available insulin patch pump. To check insurance specific coverage, visit the Omnipod site.
“When we think about why people don't adopt these technologies, despite the fact that they clearly increase Time in Range and achieve better outcomes and better quality of life, it is down to how complex and expensive they are to use,” said Insulet CEO Shacey Petrovic, emphasizing Insulet’s goals to keep costs low. “The vast majority of our users today are accessing Omnipod DASH for less than $50 a month, and we fully expect that to be the case for Omnipod 5 as well.”
For the more than 250,000 current Omnipod users, Insulet also offers a program called OmnipodPromise that allows any new or existing user of the Omnipod DASH device to upgrade to the Omnipod 5 system free of charge.
“People interested in a tubeless technology can try Omnipod DASH free for 30 days now and they will be easily upgraded to Omnipod 5 with a prescription directly to their pharmacy once available and covered by insurance,” said Wiczek.
What features are coming for the Omnipod 5 system?
Insulet said they are working on the development of the iOS version of the Omnipod 5 app.
Insulet is also working to expand the availability of their AID system to even more people with diabetes. “We will soon submit for the pre-school group, aged 2 to 6 years, a group for which we have impressive pivotal data,” said Wiczek. “In addition, we are currently conducting a clinical study with Omnipod 5 in people with type 2 diabetes.”
Though the system is currently designed to only work with the Dexcom G6 CGM, integration with the Dexcom G7 CGM (which is not yet FDA cleared) is in development. Additionally, Insulet and Abbott have a partnership, so there is hope that integration with the Freestyle Libre CGMs may be in the pipeline.
The bottom line
The Omnipod 5 was submitted for FDA clearance at the beginning of 2021, but due to delays at the FDA, the system was cleared just recently.
“The one good thing about the extra time that it's taken us [to get FDA clearance] is now we have 200,000 days of user data, almost two years on the system for people,” said Petrovic. “From that data, we know that the system is a life-changing technology for people living with diabetes, and we're going to work very hard to make it as accessible and available to everybody as rapidly as we can and could not be more excited to be here in this moment.”
To learn more about the Omnipod 5, its features, and its availability, visit Insulet’s FAQ page here.








