The Biggest News in Diabetes Technology, Drugs, and Nutrition: Highlights from ADA 2019

The diaTribe team was on the ground at the 2019 ADA 79th Scientific Sessions to share several of the greatest highlights from the conference!
The American Diabetes Association (ADA) 79th Scientific Sessions was full of exciting news on advances and studies in diabetes technology, treatments, and nutrition. Click on the links below to learn more!
Diabetes Technology
- Tandem’s Control-IQ Automated Insulin Delivery System Improves Time-in-Range and Lowers A1C Compared to Using Pump with CGM
- CITY Trial Supports Use of CGM in Adolescents with Type 1 Diabetes
- Dr. Roy Beck on CGM Accuracy and Use: “The benefit is far exceeding the burden now.”
- Medtronic and Dexcom Have Become Official Tidepool Loop Partners!
- Medtronic MiniMed 780G System and Next-Gen CGM Clinical Trials Now Enrolling Participants
-
Beta Bionics Gen 3 iLet Device Improves Time-in-Range By 2 Hours Per Day
- First Eversense Real-World Data in US: Accuracy Decline and 75% Re-Inserted; Plus, No Fingersticks!
- International Consensus on Time-in-Range Outlines CGM-Based Targets
-
Omnipod Horizon Automated Insulin Delivery System in 2-6-Year-Olds Improves Time-in-Range
-
CGM Helps Older Adults with Type 1 Diabetes Reduce Hypoglycemia and Improve Time-in-Range
Diabetes Drugs
- PIONEER 6 Shows Oral (Pill) Ozempic Is Safe For Heart In Addition to Weight Loss and A1C Reduction
- CAROLINA Finds No Differences Between Tradjenta and Glimepiride Heart Health Outcomes
- Teplizumab: First Drug Shown to Delay Type 1 Diagnosis
- Heart and Kidney Benefits from Once-Weekly GLP-1 Trulicity in People with Type 2 Diabetes
- Ultra-Rapid Lispro (URLi) Versus Humalog in Type 1 and Type 2 Diabetes
- Pill Version of Ozempic Leads to A1C Reduction and Weight Loss
- Chiglitazar Reduces A1C in People with Type 2 Diabetes: Phase 3 Study Results
- 21-Year Follow Up of STENO-2 Participants Shows 69% Reduction in Stroke and Additional 8 Years of Life Expectancy
Nutrition and Exercise
- Packed Session on the ADA Nutrition Therapy Consensus Report!
- Does When We Eat Matter? Effects of Timing on Weight, Metabolic Risk Factors, and Glycemic Control
- Weight Loss in Type 2 Diabetes Improves Beta Cell Function
- Benefits of Eating Low-Carb with Diabetes
- PREVIEW Study Confirms Lifestyle Changes Can Prevent Type 2 Diabetes, But Half Dropped Out of Study
- The Vitamin D and Type 2 Diabetes (D2d) Study — A Trial for Diabetes Prevention
Access to Care
Diabetes Technology
Tandem’s Control-IQ Automated Insulin Delivery System Improves Time-in-Range and Lowers A1C Compared to Using Pump with CGM
Tandem’s Control-IQ showed very positive results at ADA – the session ended with multiple audience members expressing their excitement about using this automated insulin delivery system as soon as it becomes available. As a reminder, Control-IQ is an automated insulin delivery system that combines the Dexcom G6 CGM, Tandem’s t:slim X2 pump, and an algorithm.
In participants 14-71 years old:
-
Time-in-range (TIR) increased by 2.6 hours (70% time-in-range compared to 59%), with the most benefit from reducing time spent in hyperglycemia (above 180 mg/dl)
-
Control-IQ helped participants reduce A1C from a baseline of 7.4%
-
Average blood glucose levels were about 14 mg/dl lower with Control-IQ (156 mg/dl vs. 170 mg/dl)
-
Time below 70 mg/dl was below 2% per day in both groups
-
Time in closed-loop (akin to auto-mode on 670G) was 92%
Importantly, the TIR benefits came early – in the first month of use! – and were maintained across the six-month study period.
Control-IQ also achieved near-perfect scores on a technology acceptance questionnaire. People rated ease of use as 4.7/5, usefulness as 4.6/5, trust as 4.5/5, and desire to continue using as 4.8/5. These positive ratings speak to the system’s convenience: no modes to juggle, no fingersticks due to the Dexcom G6 CGM, and limited alarms. Impressively, 100% of participants stayed in the study.
Tandem confirmed that Control-IQ will be submitted to the FDA for 14 years and up, with a US launch expected “this year, subject to FDA approval.” A study in younger children (ages 6 and up) is up next.
CITY Trial Supports Use of CGM in Adolescents with Type 1 Diabetes
The CITY trial tested whether the Dexcom G5 continuous glucose monitor (CGM) could improve outcomes in adolescents (14-25 years) with type 1 diabetes. Compared to BGM, CGM resulted in a 0.4% greater reduction in A1C after six months – impressive given that meeting A1C goals is particularly difficult in this age group. (The average starting A1C was 8.9%.) Those on CGM also experienced 1.4 hours per day more in-range (70-180 mg/dl), slightly less time spent above 180 mg/dl (58% vs. 54% of the day), and less time spent below 70 mg/dl (3.2% vs. 2.2%). These benefits may not seem like a lot, but it’s an hour less time high a day, and 15 minutes less time low a day.
While these results are exciting, Dr. Lori Laffel of the Joslin Diabetes Center emphasized that time spent above 180 mg/dl still comprised over half the day, indicating that more work needs to be done. Encouragingly, good news came from the user reports, which detailed more satisfaction with CGM than BGM and greater perceived benefits of CGM, to name a few. We’re also happy to see that there were no differences in diabetes distress or sleep quality between the CGM and BGM groups – a concern due to CGM alarms.
Participants from both groups are currently being followed for an additional six months using the Dexcom G6. This is such exciting research and we’re thrilled at these outcomes. No matter what your age, if you take insulin for meals and are not on CGM, ask your healthcare provider about it and mention this study!
Dr. Roy Beck on CGM Accuracy and Use: “The benefit is far exceeding the burden now.”
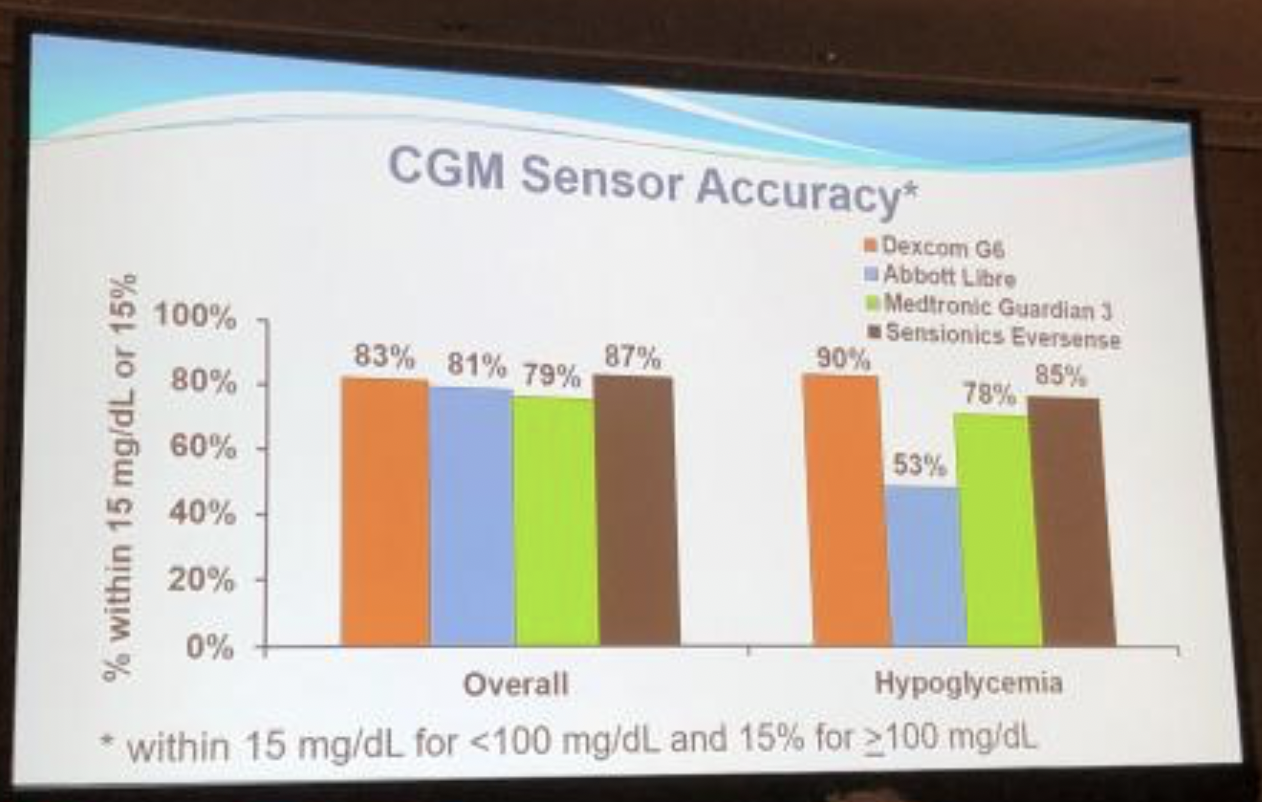
Jaeb Center’s Dr. Roy Beck provided a very positive overview of continuous glucose monitor (CGM) options in the US, covering the four available systems: Dexcom’s G6, Abbott’s FreeStyle Libre, Medtronic’s Guardian 3, and Senseonics’ Eversense.
We appreciated seeing the system-by-system accuracy stacked up side-by-side. The main takeaway: all four CGMs are pretty comparable on overall accuracy (left side of photo). There were some differences in accuracy of hypoglycemia (right side of photo), with the G6 being the most accurate in this study.
Dr. Beck concluded that CGM is becoming more widespread, and the future will see sensors that are smaller, disposable, and cheaper – facilitating more widespread use, particularly in type 2 diabetes.
Medtronic and Dexcom Have Become Official Tidepool Loop Partners!
 In new news, Medtronic announced that its Bluetooth-enabled alternate controller enabled (ACE) pump and Guardian Sensor 3 interoperable continuous glucose monitor (iCGM) will be compatible with the Tidepool Loop automated insulin delivery (AID) app. Medtronic’s Bluetooth-enabled ACE pump and Guardian Sensor 3 iCGM are both under development and not yet submitted to FDA. Dexcom has also partnered with Tidepool Loop to integrate its already-available G6 iCGM.
In new news, Medtronic announced that its Bluetooth-enabled alternate controller enabled (ACE) pump and Guardian Sensor 3 interoperable continuous glucose monitor (iCGM) will be compatible with the Tidepool Loop automated insulin delivery (AID) app. Medtronic’s Bluetooth-enabled ACE pump and Guardian Sensor 3 iCGM are both under development and not yet submitted to FDA. Dexcom has also partnered with Tidepool Loop to integrate its already-available G6 iCGM.
Tidepool Loop will be an automated insulin delivery app for iPhone and Apple Watch that connects to an insulin pump and CGM using Bluetooth and automatically adjusts the user’s basal rate with the goal to reduce high and low blood glucose.
Medtronic and Dexcom join Tidepool Loop’s first partner, Insulet’s Omnipod, leaving just Tandem, Abbott, and Senseonics as US players not yet on board. Tidepool Loop’s Medtronic and Dexcom partnerships are a huge step in developing devices that can be used together and increasing the number of options for people with diabetes.
Medtronic MiniMed 780G System and Next-Gen CGM Clinical Trials Now Enrolling Participants
The MiniMed 780G is the next iteration of the 670G hybrid closed loop system. Improvements and new features include automatic correction boluses, Bluetooth connectivity, remote software updating, a time-in-range goal greater than 80%, and an adjustable target blood glucose level down to 100 mg/dl. It will be enrolling up to 350 people with type 1 diabetes ages seven and up. See more information here!
Medtronic’s next-gen CGM will only require calibration on day one of the seven-day sensor. It will be enrolling up to 460 people with both type 1 and type 2 diabetes ages two and up. Read more here.
Beta Bionics Gen 3 iLet Device Improves Time-in-Range By 2 hours Per Day
 Beta Bionics’ Gen 3 iLet device is an automated insulin delivery system that combines the iLet insulin pump, an algorithm to adjust insulin delivery, and a continuous glucose monitor (CGM). In this seven-day study, participants used either the Dexcom G5 or Senseonics 90-day Eversense CGM. Both pump and multiple daily injection (MDI) users were included in the study.
Beta Bionics’ Gen 3 iLet device is an automated insulin delivery system that combines the iLet insulin pump, an algorithm to adjust insulin delivery, and a continuous glucose monitor (CGM). In this seven-day study, participants used either the Dexcom G5 or Senseonics 90-day Eversense CGM. Both pump and multiple daily injection (MDI) users were included in the study.
Compared to usual care, time-in-range (70-180 mg/dl) improved by 1.9 hours per day on the iLet (70% versus 62%) – this was a statistically significant difference. The G5 and Eversense CGMs performed similarly, showing that the Beta Bionics system works with two different CGMs.
Excitingly, when iLet runs on a bihormonal system – that is, both insulin and glucagon – users see an even greater time-in-range benefit. In bi-hormonal mode, users spent 79% of the day in range (compared to 71% with insulin only), translating to two additional hours in range. Those using bi-hormonal mode also experienced less hypoglycemia.
Though the sample sizes of these studies were small, these results are an important step forward towards a closed loop system.
First Eversense Real-World Data in US: Accuracy Decline and 75% Re-Inserted; Plus, No Fingersticks!
Following the approval and launch of the implantable Senseonics 90-day Eversense in the US, we were excited to learn of how the CGM is performing in the real world (outside of clinical trials). Unsurprisingly, sensor accuracy declined – a common occurrence with real-world use of CGM. About 75% of people who completed their first 90 days of Eversense decided to insert their second sensor.
People spent 62% time-in-range (70-180 mg/dl), translating to about 15 hours per day and just 4% time below 70 mg/dl (about one hour per day). Curious about safety issues of implanting a sensor under the skin? Only 5% experienced skin irritation, 2% experienced mild infection at the insertion site, 2% failed to remove the sensor on the first attempt (done by healthcare provider, not user), and 2.5% experienced irritation due to the transmitter adhesive.
In other news, the FDA approved the device for non-adjunctive insulin dosing, which means that people can make dosing decisions using Eversense without confirming their blood glucose level with a fingerstick. This puts Eversense in the same approval category as the Dexcom G6 and Abbott’s FreeStyle Libre.
International Consensus on Time-in-Range Outlines CGM-Based Targets
At the 2019 Advanced Technologies & Treatments for Diabetes (ATTD), we got a preview of the international consensus report on TIR goals. The refined report has now been published online in Diabetes Care. The key takeaways were that people with type 1 and type 2 diabetes should aim to spend:
-
At least 70% of TIR (70-180 mg/dl)
-
Less than 25% of time above 180 mg/dl
-
Less than 5% of time below 70 mg/dl
Importantly, the panel of authors add that even if a person isn’t at 70% in-range, every 5% increase in TIR is significant! After all, as diaTribe Senior Editor Adam Brown points out along with many notable researchers and clinicians, every 5% increase is equal to approximately one hour or more a day spent in range.
The hope is that the recommended goals increase CGM use – until now, there had not been any agreement on what appropriate time-in-range goals should be. We were particularly happy that this working group was patient-oriented – diaTribe Editor-in-Chief Kelly Close was a part of the author group as were other renowned researchers with diabetes, including JDRF CEO Dr. Aaron Kowalski and Dr. Irl Hirsch of the University of Washington.
Omnipod Horizon Automated Insulin Delivery System in 2-6-Year-Olds Improves Time-in-Range
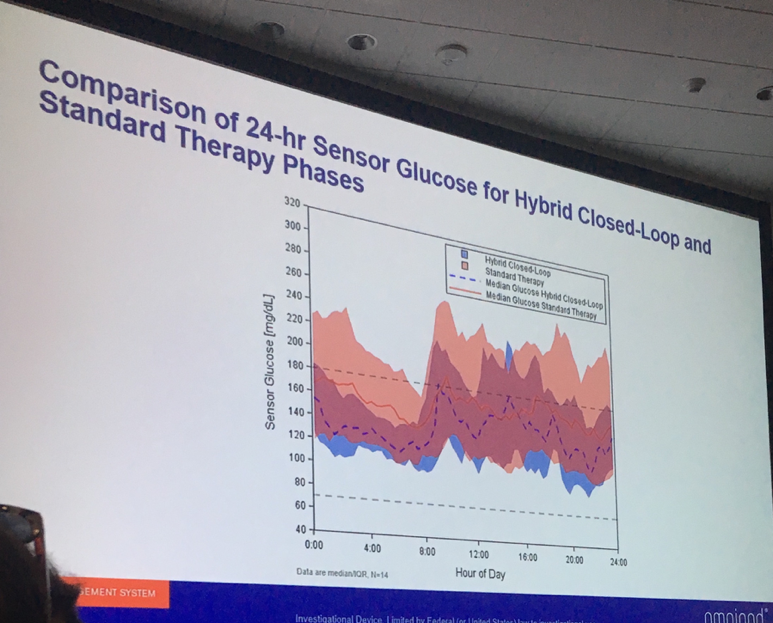 Dr. Bruce Buckingham (Stanford University) presented strong data from a three-day study of Insulet’s Omnipod Horizon automated insulin delivery system in children ages 2-6 with type 1 diabetes. Overall, Horizon use resulted in a remarkable four more hours per day spent in range (70-180 mg/dl) – 73% vs. 55%. This impressive improvement was primarily attained primarily because the children spent less time above 180 mg/dl. Average glucose levels fell from 172 mg/dl with standard therapy to 148 mg/dl on the Horizon system. The system performed well despite ample exercise (laser tag, a farm visit, jumping on a trampoline, etc.) and meals, which make performance in an automated system more challenging.
Dr. Bruce Buckingham (Stanford University) presented strong data from a three-day study of Insulet’s Omnipod Horizon automated insulin delivery system in children ages 2-6 with type 1 diabetes. Overall, Horizon use resulted in a remarkable four more hours per day spent in range (70-180 mg/dl) – 73% vs. 55%. This impressive improvement was primarily attained primarily because the children spent less time above 180 mg/dl. Average glucose levels fell from 172 mg/dl with standard therapy to 148 mg/dl on the Horizon system. The system performed well despite ample exercise (laser tag, a farm visit, jumping on a trampoline, etc.) and meals, which make performance in an automated system more challenging.
When it launches, Horizon will be made up of the Omnipod tubeless patch pump, the Dexcom G6 CGM, and an algorithm. In this study, an earlier Dexcom model was used. The Horizon pivotal study (collecting data to submit to regulatory agencies like the FDA) is set to begin toward the end of 2019.
CGM Helps Older Adults with Type 1 Diabetes Reduce Hypoglycemia and Improve Time-in-Range
The WISDM study examined the impact of CGM use in people with type 1 above the age of 60 years. Participants were using either the Dexcom G5 CGM or standard blood glucose meters (BGM). The CGM group spent less time in hypoglycemia (below 70 mg/dl) and two more hours per day in range (70-180 mg/dl) by the end of the six months compared to the BGM group. A1C reduction was also greater in the CGM group. Importantly, those on CGM reported significantly fewer severe hypoglycemia events (defined as requiring assistance of another person). These benefits were seen regardless of pump versus multiple daily injection (MDI) usage.
While there has been doubt about the effectiveness of diabetes technology in older populations, the WISDM study shows that CGM may be just as important for older adults as younger adults. As CGMs become even easier to use and affordable in the coming years, they should be adopted by more and more people of all ages and backgrounds.
Click here to jump back to the Table of Contents.
Diabetes Drugs
PIONEER 6 Shows Oral (Pill) Ozempic Is Safe For Heart In Addition to Weight Loss and A1C Reduction
We were highly anticipating the full PIONEER 6 results following the release of initial results that showed a 21% risk reduction of heart attack, stroke, and heart-related death. The exhibition hall was packed this morning as results came out! This study compared an oral version of Ozempic (semaglutide), a GLP-1 agonist, against placebo (a “nothing” pill).
It was a very exciting set of presenations – in a population of people with type 2 diabetes and heart disease or risk factors for heart disease, compared to placebo, oral Ozempic:
-
Reduced risk of heart-related death by 51%
-
Did not have a meaningful difference in heart attacks
-
Did not have a meaningful difference in strokes
-
Reduced A1C by 1.0% from a starting A1C of 8.2%
-
Reduced weight by about 9 lbs (4.2 kg) from a starting weight of 200 lbs (91 kg) – this is nearly 5% of body weight lost, which is very meaningful
Overall, PIONEER 6 was exciting and further paves the way for the first oral GLP-1 pill to become available for people with diabetes. Presenters were hopeful that taking a pill rather than injecting may remove barriers to use in many. Notably, oral Ozempic has some fasting requirements – people have to take oral Ozempic on an empty stomach and then wait 30 minutes before eating – and we will see how people with diabetes feel about this when they get closer to approval and launch! For some, it may well be a meaningful hurdle while others will probably sail by this.
Oral Ozempic is currently under review by the FDA and other regulatory agencies. See here for the session slides from the notable Dr. John Buse at UNC, and here for the article published in the New England Journal of Medicine.
CAROLINA Finds No Differences Between Tradjenta and Glimepiride Heart Health Outcomes
Previous studies have suggested that a type of medication called sulfonylureas, particularly Orinase (tolbutamide), may be unsafe for the heart. The FDA currently has a product-label warning for heart-related death for all sulfonylureas.
New CAROLINA study results, however, demonstrated that the DPP-4 inhibitor Tradjenta (linagliptin) and the sulfonylurea glimepiride are equally safe for the heart in people with type 2 diabetes. The CAROLINA trial looked specifically at non-fatal heart attacks, non-fatal strokes, and heart-related death. Since we have evidence that Tradjenta is no more dangerous than placebo (a “nothing” pill), there’s reason to believe that glimepiride also poses no heart safety risk – though we don’t know if this applies to other sulfonylureas like glipizide, gliclazide, and glibenclamide.
Interestingly, although sulfonylureas are generally associated with weight gain, the trial also showed no difference between Tradjenta and glimepiride on changes in weight. Participants lost an average of about 1-3 pounds in both groups – because people were really “looked after” in the trials, it’s unlikely that this weight loss would happen with glimepiride in the real world. There were also no significant differences in A1C reduction; both groups experienced an initial drop in A1C that creeped back up to the starting level of 7.1%.
However, sulfonylureas are known to carry a greater risk of hypoglycemia than other type 2 diabetes medications. CAROLINA confirmed increased hypoglycemia with glimepiride, showing a 77% increased risk of hypoglycemia overall; 38% of those on glimepiride experienced hypoglycemia, compared to 11% of those on Tradjenta. Looking more closely, glimepiride had an 85% increased risk of severe hypoglycemia (low blood sugar requiring assistance from a third party) and a 93% increased risk of hospitalization for hypoglycemia. This alone is reason to avoid glimepiride if at all possible. If not, we encourage people to check blood glucose levels as frequently as possible.
Though Tradjenta clearly has the upper hand on hypoglycemia, those who are on glimepiride due to its lower cost can be more assured about heart safety.
Teplizumab: First Drug Shown to Delay Type 1 Diagnosis
In phenomenal news for the diabetes community, teplizumab delayed type 1 diagnosis by two years compared to placebo (“nothing” treatment). On average, time to diagnosis for the teplizumab group was four years, compared to two years with placebo. At the end of the trial, there were an impressive 53% in the teplizumab-treated group who did not have type 1 diabetes, as compared to 28% in the placebo group. While not a cure, two years of living without the daily burden of diabetes management is a meaningful outcome.
Teplizumab is an anti-CD3 treatment that has been previously shown to reduce the loss of beta cell function by suppressing the body’s immune system. The study enrolled 76 relatives – both adults and children – of people with type 1 diabetes; the participants did not have type 1 but were at high risk for developing the disease as they had elevated blood sugar levels and two or more type 1 autoantibodies (indicators of immune system attack on beta cells). People took teplizumab for 14 days (as an IV infusion) at the beginning of the trial.
The compound was first developed by Dr. Jeffrey Bluestone of UCSF in 1986. After a long history of both positive and negative outcomes, teplizumab has been acquired by Provention Bio for continued development.
Heart and Kidney Benefits from Once-Weekly GLP-1 Trulicity in People with Type 2 Diabetes
The REWIND trial examined the heart effects of Trulicity, a once-weekly injectable GLP-1 agonist for people with type 2 diabetes. It revealed a 12% reduced risk of a combination of non-fatal stroke, non-fatal heart attack, and heart-related death compared to placebo (a “nothing” pill). This benefit was the same among study participants who had existing heart disease and those who only had heart disease risk factors. As such, REWIND offers the first evidence that GLP-1 agonists may prevent strokes and heart attacks in people without diagnosed heart disease.
Looking more closely at the heart benefits, a reduction in non-fatal strokes appears to have driven much of Trulicity’s impact, with a 24% risk reduction compared to placebo. The other two components, non-fatal heart attack and heart-related death, were non-significant, meaning that the reduced number of these events could have been due to chance.
When the trial started, participants had lower A1C’s than in other studies (a median of 7.2%), and a lower number of them had existing heart disease (31%). This makes the results even more meaningful because an even broader, healthier group saw prevention benefits. Notably, REWIND is also the longest heart outcomes trial for a GLP-1 agonist at over five years in length. For these reasons, the study sponsors suggest that its results are closer to reflecting the “real world” than those of other studies examining Victoza, Ozempic, Adlyxin/Lyxumia, and Bydureon.
The trial also showed potential positive kidney effects for Trulicity. The risk of a combination of kidney outcomes (new macroalbuminuria, 30% decrease in kidney filtration rate, and progression to kidney replacement) was reduced by 15% compared to placebo. This was driven by a 23% relative risk reduction for new macroalbuminuria (large amounts of protein in the urine, suggesting the kidneys are not working properly); the other two components were not significant. Emerging evidence suggests that another class of drug, SLGT-2 inhibitors (Invokana, Farxiga, Jardiance, and Steglatro), may have even stronger kidney benefits.
Previous studies have demonstrated the A1C-lowering and weight loss effects of Trulicity.
Ultra-Rapid Lispro (URLi) Versus Humalog in Type 1 and Type 2 Diabetes
URLi (pronounced “early”) is an ultra-rapid acting mealtime insulin in development by Lilly. It is a modified version of Humalog that gives it a faster onset and offset profile.
In type 1 diabetes (PRONTO-T1D study), compared to Humalog, URLi:
-
Reduced post-meal blood glucose levels by 28 mg/dl at one hour and 31 mg/dl at two hours
-
Did not have a difference in A1C (7.21% vs. 7.29%)
-
Did not have a significant difference in severe hypoglycemia, but reduced hypoglycemia events past the four-hour mark
Notably, post-meal URLi was also studied – taking the mealtime insulin after a meal instead of before. With post-meal URLi, the blood glucose level benefit was lost, but the presenter noted if necessary, URLi could be used after a meal.
In type 2 diabetes (PRONTO-T2D study), compared to Humalog, URLi:
-
Reduced post-meal blood glucose levels by 12 mg/dl at one hour and 17 mg/dl at two hours
-
Did not have a difference in A1C (6.92% vs. 6.86%)
-
Had a slightly higher frequency of hypoglycemia both 1-2 hours and 2-4 hours after a meal
Pill Version of Ozempic Leads to A1C Reduction and Weight Loss
Two trials examined the effectiveness of a once-daily pill (oral) version of Ozempic (semaglutide) for lowering A1C and weight in people with type 2 diabetes. Oral Ozempic is the first non-injectable GLP-1 agonist.
The PIONEER 2 trial compared oral Ozempic to once-daily SGLT-2 inhibitorJardiance in people already taking metformin, and with an A1C between 7% and 10.5%. The study found:
-
After about six months, A1C had decreased by an average of 0.4% more in the oral Ozempic group (1.3%) than in the Jardiance group (0.9%). These A1C drops were maintained after one year.
-
After about six months, people on both Ozempic and Jardiance lost about 8 pounds on average. While both medications resulted in significantly more weight loss than metformin alone, the difference between weight loss with Ozempic or Jardiance was not significant. The weight loss was maintained after one year.
-
The group taking oral Ozempic had more negative side-effects leading people to discontinue the trial early – mainly nausea, vomiting, and abdominal pain. 20% of people taking oral Ozempic experienced nausea, versus 2% of people taking Jardiance.
The PIONEER 4 trial compared oral Ozempic to once-daily injectable GLP-1 agonist Victoza in people already taking metformin, and with an A1C between 7 and 9.5%. The study found:
-
After one year, oral Ozempic had lowered A1C more than Victoza (1.2% vs. 0.9% on average); this difference was statistically significant, meaning not due to chance.
-
After six months, oral Ozempic resulted in a 9.7-pound average weight loss and Victoza resulted in a 6.8-pound average weight loss. This was maintained after one year.
-
There were a higher number of side-effects leading people to discontinue oral Ozempic early – mainly nausea and diarrhea. 44% of the oral Ozempic group experienced these gastrointestinal side-effects, versus 34% of the Victoza group and 24% of the group not taking a GLP-1 agonist.
Oral Ozempic was submitted to the FDA for approval in March 2019, and a decision is expected by September 2019.
Chiglitazar Reduces A1C in People with Type 2 Diabetes: Phase 3 Study Results
In two phase 3 studies in type 2 diabetes, chiglitazar proved to be safe and showed greater A1C reductions compared to placebo and comparable effects to sitagliptin (Januvia), a DPP-4 inhibitor. Chiglitazar is a PPAR Pan-Agonist that activates a protein that regulates genes involved in using fats for energy and decreasing insulin resistance.
In the CMAP trial comparing chiglitazar to placebo, both the 32 mg and 48 mg doses of chiglitazar showed a greater decrease in A1C lowering at 24 weeks than placebo. Over 24 weeks:
- The 32 mg dose of chiglitazar was associated with an A1C decrease of 1.3% (from a baseline A1C of 8.5%).
- The 48 mg dose of chiglitazar was associated with an A1C decrease of 1.5% (from a baseline A1C of 8.6%).
- The placebo group experienced an A1C decrease of 0.5% (from a baseline A1C of 8.6%).
In the CMAS trial comparing chiglitazar to Januvia, chiglitazar achieved similar A1C lowering to Januvia at 24 weeks. Over 24 weeks:
- The 32 mg dose of chiglitazar was associated with an A1C decrease of 1.4% (from a baseline A1C of 8.5%).
- The 48 mg dose of chiglitazar was associated with an A1C decrease of 1.5% (from a baseline A1C of 8.6%).
- Januvia was associated with an A1C decrease of 1.5% (from a baseline A1C of 8.6%).
Notably, chiglitazar proved to be safe for people with type 2 diabetes, with no difference in side effects compared to placebo or Januvia. In less positive news from the trial, chiglitazar was associated with slight weight gain (about 2.2lbs or 1 kg).
21-Year Follow Up of STENO-2 Participants Shows 69% Reduction in Stroke and Additional 8 Years of Life Expectancy
STENO-2 followed people with type 2 diabetes for over two decades to compare outcomes of receiving multifactorial interventions or standard of care. Multifactorial interventions were simultaneous glucose, blood pressure, and lipid lowering over a period of eight years.
Impressively, those receiving the more intensive care had a 69% reduced risk for stroke; 26% in the standard of care group had a stroke, compared to 11% in the intensive care group. The intensive care resulted in an extra eight years of life expectancy.
Though a relatively small trial (160 participants), the long-term and consistently compelling results provide clinical and economic reasons for meeting glucose, blood pressure, and lipids. Dr. Peter Gæde of the Steno Diabetes Center noted that while the intervention is costly in the short-term, it is a very smart investment due to the powerful data on the prevention of complications.
Click here to jump back to the Table of Contents.
Nutrition and Exercise
Packed Session on the ADA Nutrition Therapy Consensus Report!
 Though we covered the report and its recommendations when they came out, we were eager to hear from guideline authors. Dr. William Yancy summarized the key takeaways of the report:
Though we covered the report and its recommendations when they came out, we were eager to hear from guideline authors. Dr. William Yancy summarized the key takeaways of the report:
-
Eat more non-starchy vegetables, which include salad greens, broccoli, cauliflower, and more listed here
-
For type 2 diabetes, reducing overall carbohydrate intake as well as the Mediterranean has the most evidence for improving blood sugar levels
-
For type 1 diabetes, while no eating pattern has robust evidence, low-carb has preliminary positive evidence
-
For prediabetes, low-fat has the most evidence for benefit, followed by Mediterranean and low-carb diets
Diabetes advocate Kelly Rawlings highlighted the importance of individualized strategies. Some specific examples she gave were:
-
Try different cooking methods to enjoy vegetables (sautéed, steamed, fresh, roasted, etc.); for some vegetable inspiration, see here for recipes
-
Eat a hamburger without the bun
Dr. Christopher Gardner concluded the session by asking people to focus on decreasing sugar consumption, saying that “America is the leading consumer of added sugars. Let’s not bicker about paleo, keto, etc…let’s focus on this!”
Does When We Eat Matter? Effects of Timing on Weight, Metabolic Risk Factors, and Glycemic Control

Experts shared the latest data behind intermittent fasting and meal timing. This was one of the most crowded sessions of the day – highlighting the quickly growing interest surrounding meal timing and intermittent fasting!
The experts shared that time-restricted feeding (TRF) – eating in a narrower time period each day, typically within an 8-10 hour window – can improve health. The strongest evidence is from “early TRF”, e.g. eating from 8am - 6pm as opposed to 4pm - 2am, because it better aligns eating with the body’s circadian rhythm. Early TRF is associated with:
-
Decreased hunger or food intake
-
Weight loss
-
Reduced glucose and insulin levels and improved insulin sensitivity
-
Lowered blood pressure, and
-
Increased fat oxidation (i.e., fat burning)
It was emphasized that TRF can be practiced with or without cutting calories. In addition, benefits are proportional to daily fasting duration; this means even small modifications in one’s eating produce results, but a longer fasting time may result in greater health benefits.
Experts stressed, however, that the benefits of changing meal timing do not outweigh the benefits of reducing processed food intake. Dr. Courtney Peterson from University of Alabama at Birmingham stated, “despite the evidence for the benefits of intermittent fasting, I still don’t subscribe to the ‘you can eat whatever you want’ theory.” Speak with your healthcare provider if you are interested in time-restricted feeding, as there may be necessary adjustments to your insulin requirements and diabetes medications.
Weight Loss in Type 2 Diabetes Improves Beta Cell Function
 The Diabetes Remission Clinical Trial (DiRECT) tested whether restricting calories consumed would lead to remission in type 2 diabetes, defined as having an A1C below 6.5% and discontinuing all diabetes medications for at least two months. After one year, 46% of participants achieved remission; after two years, of the 46%, 70% were able to maintain remission.
The Diabetes Remission Clinical Trial (DiRECT) tested whether restricting calories consumed would lead to remission in type 2 diabetes, defined as having an A1C below 6.5% and discontinuing all diabetes medications for at least two months. After one year, 46% of participants achieved remission; after two years, of the 46%, 70% were able to maintain remission.
New analysis of the DiRECT data showed that people who put their diabetes into remission had full recovery of beta cell function: the insulin secretion level in response to hyperglycemia (high blood sugar) was indistinguishable from people who did not have type 2 diabetes.
How does losing weight improve beta cell function? Professor Roy Taylor says that it is specifically reducing fat in the liver and pancreas that improves both insulin secretion by the beta cells as well as insulin sensitivity.
There is now evidence to show that type 2 diabetes does not have to be a progressive disease, if action is taken early on. In a sentence, Professor Taylor summarized the results as “good news for people with type 2 diabetes,” and we couldn’t agree more!
If you would like to learn more about type 2 diabetes remission, click here.
Benefits of Eating Low-Carb with Diabetes
The new ADA nutrition guidelines outline low-carb diets as a viable approach for managing diabetes. (In diaTribe’s own nutrition principles, we encourage readers to limit meals down to 30 grams of carbs.) In this session, experts shared potential benefits of eating a low-carb diet when living with diabetes, including:
-
Reduced A1C and glucose levels
-
Lower medication use (and therefore cost)
-
Weight loss
-
Lowered blood pressure
-
Reduced inflammation
-
Reduced food cravings and hunger
Experts also addressed common concerns of the low-carb approach: cholesterol, ability to stick to low-carb, and taste appeal. In two trials mentioned, individuals with diabetes on a low-carb diet had increased LDL cholesterol (the “bad” kind). However, all other cardiovascular risk factors, including triglycerides, significantly improved. To test the ability for people to stay on a low-carb diet, researchers studied low-fat and low-carb plans and found no difference. Lastly, Dr. Laura Saslow emphasized that low-carb diets can be delicious! See one of our most well-read pieces for a few Low-Cost, Low-Carb recipes.
Because of increased mealtime insulin resistance with low-carb diets, an insulin to carbohydrate ratio may not be sufficient for bolusing. If you are trying a low-carb or very low-carb diet, presenters recommended discussing an insulin to protein ratio instead with your healthcare provider.
PREVIEW Study Confirms Lifestyle Changes Can Prevent Type 2 Diabetes, But Half Dropped Out of Study
PREVIEW is the largest randomized control trial (gold standard of clinical trials) to date on the prevention of type 2 diabetes in adults with prediabetes through diet and exercise. Participants were in one of four groups: 1) high protein, low-glycemic index (GI) diets + moderate physical activity, 2) high-protein, low-GI diets + high-intensity physical activity, 3) moderate protein, moderate-GI diets + moderate physical activity, and 4) moderate protein, moderate-GI diets + high-intensity physical activity.
After three years, just 4% of those who remained in the study were diagnosed with type 2 diabetes. There were no differences between diets or exercise regimes. Researchers estimated that without any intervention, about 21% would have been diagnosed with type 2 diabetes. Dr. Ian MacDonald believes that the comparable results across the four groups was due to the initial and sustained weight loss throughout the trial. Before people were randomly assigned to a group, everyone was put on a low-calorie diet, with an average weight loss of 23 lbs (10.7 kg) from an average starting weight of 220 lbs (100 kg).
Lifestyle change, however, is notoriously difficult to keep up. Only 43% of the participants initially enrolled in PREVIEW completed the three-year program, highlighting the importance of coaching and support.
The Vitamin D and Type 2 Diabetes (D2d) Study — A Trial for Diabetes Prevention
Does vitamin D supplementation prevent type 2 diabetes? The D2d trial found that supplementation with vitamin D3 (the natural form of vitamin D that your body makes from sunlight) did not delay progression from prediabetes to type 2 diabetes.
2,423 people with prediabetes were given 4,000 IU of vitamin D per day for two years. The rate of progression from prediabetes to type 2 diabetes in the D2d trial was:
-
9.4 type 2 diagnoses for every 100 person-years in people taking vitamin D; and
-
10.7 type 2 diagnoses for every 100 person-years with placebo (a “nothing” pill).
In other words, for every 100 people followed for 1 year, 9.4 progressed to type 2 diabetes when taking vitamin D and 10.7 progressed to type 2 diabetes with placebo.
While these results show a lower rate of progression to type 2 diabetes in the vitamin D group, they were not statistically significant, meaning the difference could be due to chance.
In positive news, the study showed that this high level of vitamin D supplementation is safe. Dr. Anastassios Pittas, who presented these results, explained that there has been concern over toxicity with very high vitamin D levels. He said 4,000 IU/day is considered the upper limit of safe supplementation. D2d serves as the best evidence to date on the long-term safety of high vitamin D supplementation.
Click here to jump back to the Table of Contents.
Access to Care
Looking to Reduce Copays? Cut the Higher Dose Pills in Half
Dr. Jean Francois-Yale from McGill University outlined how, in some instances, healthcare providers might prescribe the higher dose of an SGLT-2 inhibitor (or even a GLP-1 agonist) but tell people with diabetes to cut their dose in half. This leads to a lower copay for people with diabetes, a method that works because the higher and lower doses are the same price.
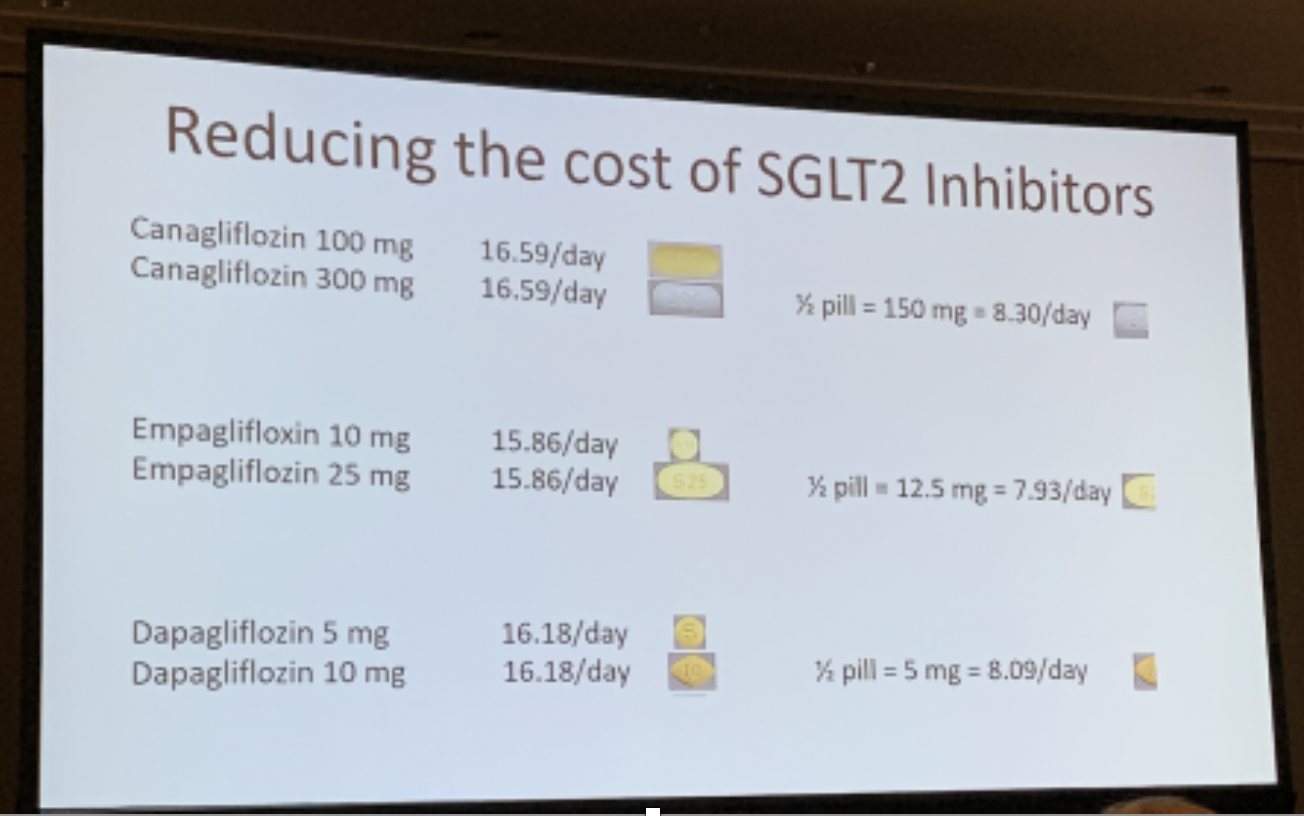
SGLT-2 inhibitors (eg. Invokana, Farxiga, Jardiance) and GLP-1 agonists (eg. Victoza, Trulicity, Ozempic) have been shown to reduce A1C, improve weight loss, and protect the heart and kidneys in people with type 2 diabetes. Dr. Francois-Yale turned to what is a big hurdle for some people for these types of drugs: cost. He detailed a simple yet novel strategy that he uses in practice that can make a dramatic difference for people with diabetes who are typically receiving the lower dose of the drug: cut high dose pills in half and take them separately. This effectively halves the price of their medication, but people with diabetes still get the dose of medicine they need.
Click here to jump back to the Table of Contents.