Do you ever wonder why there aren’t cheaper “generic” insulin products available? This may soon change as the patents for branded insulin begin to expire. In fact, the...
Diabetes Drug News
Diabetes Drugs are responsible for helping to decrease blood sugar levels so that you can try to stay in a healthier range and help avoid serious complications.
Metformin Insulin SGLT-2 GLP-1 DPP-4 Combo drugs Sulfonylureas TZDs
48 readers recommended
Last month, in early September, results from the DPP-4 inhibitor cardiovascular outcomes trials SAVOR-TIMI 53 (for Bristol Myers Squibb and AstraZeneca’s Onglyza) and...
48 readers recommended
On November 22, J&J/Janssen Cilags’ SGLT-2 inhibitor Invokana (canagliflozin) was approved in Europe. This makes Invokana the second SGLT-2 inhibitor approved in...
23 readers recommended
What it felt like to forget about diabetes for a week.
39 readers recommended
On February 11, the FDA released a Drug Safety Communication requesting data from the widely publicized cardiovascular outcomes trial for Onglyza (saxagliptin). The...
42 readers recommended
On September 12, Sanofi announced that it withdrew the FDA application for its once-daily GLP-1 agonist lixisenatide (named Lyxumia in Europe) for type 2 diabetes. It is...
46 readers recommended
On November 25, the FDA announced the removal of the major restrictions on the prescription and use of GlaxoSmithKline’s Avandia (rosiglitazone) for type 2 diabetes...
52 readers recommended
The consensus from most speakers at the workshop was that, while this possible link does require further investigation, the available evidence does not suggest any need...
41 readers recommended
How you can help to prevent type 1 diabetes…
JDRF and PureTech Launch Groundbreaking New Fund, T1D Innovations, Designed to Fund Type 1 Therapies
24 readers recommended
On Tuesday October 15, JDRF and PureTech announced the launch of T1D Innovations, an exciting new fund that aims to supply crucial funding for the development of type 1...
32 readers recommended
On December 27, Takeda announced that it would stop development of TAK-875 (fasiglifam) due to liver safety concerns – this was one of the most unfortunate announcements...
29 readers recommended
On June 5 and 6, an FDA advisory panel voted in favor of modifying the current Risk Evaluation and Modification Strategy (REMS), for GlaxoSmithKline’s (GSK) Avandia (...
43 readers recommended
On April 3, an FDA Advisory Committee voted an overwhelming 13-1 in favor of approving MannKind’s ultra-rapid-acting inhaled insulin Afrezza for type 1 diabetes. The...
47 readers recommended
A major need for the 79 million Americans with prediabetes…
42 readers recommended
On December 11, Orexigen announced the resubmission of its weight management drug Contrave (naltrexone/bupropion) to the FDA. The company has previously stated that it...
32 readers recommended
On April 29, Merck and Pfizer announced that they are entering a partnership to develop ertugliflozin for type 2 diabetes. Ertugliflozin is a sodium-glucose linked...
40 readers recommended
On April 15, the FDA approved GlaxoSmithKline’s once-weekly GLP-1 agonist Tanzeum (albiglutide) for type 2 diabetes, which makes it the second once-weekly GLP-1 agonist...
42 readers recommended
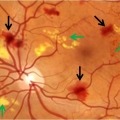
All about diabetic macular edema (DME) and three tips for avoiding it.
74 readers recommended
The top three things I’d like to see in 2014 – updates on CGM, mHealth, and combination therapy.
24 readers recommended
In May, Lexicon announced its results of LX4211, an SGLT-1/SGLT-2 inhibitor. The phase 2 trial finished its open-label, pioneer section, which showed a decrease in...